On this page
Preparing for your appointment.
To diagnose malaria, your doctor will likely review your medical history and recent travel, conduct a physical exam, and order blood tests. Blood tests can indicate:
- The presence of the parasite in the blood, to confirm that you have malaria
- Which type of malaria parasite is causing your symptoms
- If your infection is caused by a parasite resistant to certain drugs
- Whether the disease is causing any serious complications
Some blood tests can take several days to complete, while others can produce results in less than 15 minutes. Depending on your symptoms, your doctor may order additional diagnostic tests to assess possible complications.
Malaria is treated with prescription drugs to kill the parasite. The types of drugs and the length of treatment will vary, depending on:
- Which type of malaria parasite you have
- The severity of your symptoms
- Whether you're pregnant
Medications
The most common antimalarial drugs include:
- Chloroquine phosphate. Chloroquine is the preferred treatment for any parasite that is sensitive to the drug. But in many parts of the world, parasites are resistant to chloroquine, and the drug is no longer an effective treatment.
- Artemisinin-based combination therapies (ACTs). artemisinin-based combination therapy (ACT) is a combination of two or more drugs that work against the malaria parasite in different ways. This is usually the preferred treatment for chloroquine-resistant malaria. Examples include artemether-lumefantrine (Coartem) and artesunate-mefloquine.
Other common antimalarial drugs include:
- Atovaquone-proguanil (Malarone)
- Quinine sulfate (Qualaquin) with doxycycline (Oracea, Vibramycin, others)
- Primaquine phosphate
If you suspect you have malaria or that you've been exposed, you're likely to start by seeing your family doctor. However, in some cases when you call to set up an appointment, you may be referred to an infectious disease specialist. If you have severe symptoms — especially during or after travel in an area where malaria is common — seek emergency medical attention.
What you can do
Before your appointment, you might want to write down answers to the following questions:
- What are your symptoms, and when did they start?
- Where have you traveled recently?
- How long did you travel and when did you return?
- Did you take any preventive drugs related to your travel?
- What other medications do you take, including dietary supplements and herbal remedies?
Feb 09, 2023
- AskMayoExpert. Malaria. Rochester, Minn.: Mayo Foundation for Medical Education and Research; 2018.
- Jameson JL, et al., eds. Malaria. In: Harrison's Principles of Internal Medicine. 20th ed. New York, N.Y.: The McGraw-Hill Companies; 2018. https://accessmedicine.mhmedical.com. Accessed Oct. 9, 2018.
- Tintinalli JE, et al., eds. Malaria. In: Tintinalli's Emergency Medicine: A Comprehensive Study Guide. 8th ed. New York, N.Y.: McGraw-Hill Education; 2016. http://www.accessmedicine.mhmedical.com. Accessed Oct. 9, 2018.
- Malaria. Merck Manual Professional Version. http://www.merckmanuals.com/professional/infectious-diseases/extraintestinal-protozoa/malaria. Accessed Oct. 9, 2018.
- Malaria. Centers for Disease Control and Prevention. http://wwwnc.cdc.gov/travel/diseases/malaria. Accessed Nov. 6, 2015.
- Breman JG. Clinical manifestations of malaria in nonpregnant adults and children. https://www.uptodate.com/contents/search. Accessed Oct. 9, 2018.
- Daily J. Treatment of uncomplicated falciparum malaria in nonpregnant adults and children. https://www.uptodate.com/contents/search. Accessed Oct. 9, 2018.
- Key points: World malaria report 2017. World Health Organization. https://www.who.int/malaria/media/world-malaria-report-2017/en/. Accessed Oct. 9, 2018.
- Malaria. World Health Organization. https://www.who.int/malaria/en/. Accessed Oct. 9, 2018.
- Mutebi JP, et al. Protection against mosquitoes, ticks, & other arthropods. In: CDC Yellow Book 2018: Health Information for International Travelers. https://wwwnc.cdc.gov/travel/yellowbook/2018/the-pre-travel-consultation/protection-against-mosquitoes-ticks-other-arthropods. Accessed Oct. 27, 2018.
- Diseases & Conditions
- Malaria diagnosis & treatment
News from Mayo Clinic
More Information
- Malaria transmission cycle
Products & Services
- A Book: Mayo Clinic Family Health Book, 5th Edition
- Newsletter: Mayo Clinic Health Letter — Digital Edition
CON-XXXXXXXX
Your gift holds great power – donate today!
Make your tax-deductible gift and be a part of the cutting-edge research and care that's changing medicine.
Thanks for visiting! GoodRx is not available outside of the United States. If you are trying to access this site from the United States and believe you have received this message in error, please reach out to [email protected] and let us know.
Dosing Recommendations for Prevention and Treatment of Malaria
You are using an outdated browser. Upgrade your browser today or install Google Chrome Frame to better experience this site.
What is malaria?
Who is at risk, what can travelers do to prevent malaria, after travel, more information.
Malaria is a disease caused by a parasite. Mosquitoes spread the parasite to people when they bite them.
Malaria symptoms usually appear within in 7 to 30 days but can take up to one year to develop. Symptoms may include high fevers and shaking chills, flu-like illness. Without treatment, malaria can cause severe illness and death.
The mosquitoes that spread malaria are found in Africa, Central and South America, parts of the Caribbean, Asia, Eastern Europe, and the South Pacific (See maps: Eastern Hemisphere and Western Hemisphere ). Travelers going to these countries may get bit by mosquitoes and get infected.

Where are you going?
About 2,000 cases of malaria are diagnosed in the United States annually, mostly among returned travelers.
Travelers can protect themselves from malaria by taking prescription medicine and preventing mosquito bites. There is no malaria vaccine.
Take Malaria Medicine
Check your destination to see if you should take prescription malaria medication. Depending on the medicine you take, you will need to start taking this medicine multiple days before your trip, as well as during and after your trip. Talk to your doctor about which medicine you should take .
Travelers should also take steps to prevent mosquito bites.
Use an EPA-registered insect repellent

- Picaridin (known as KBR 3023 and icaridin outside the US)
- Oil of lemon eucalyptus (OLE)
- Para-menthane-diol (PMD)
- 2-undecanone
Find the right insect repellent for you by using EPA's search tool .

- Dress your child in clothing that covers arms and legs.
- Cover strollers and baby carriers with mosquito netting.
- Always follow label instructions.
- Do not use products containing oil of lemon eucalyptus (OLE) or para-menthane-diol (PMD) on children under 3 years old.
- Adults: Spray insect repellent onto your hands and then apply to a child’s face.
- If also using sunscreen, always apply insect repellent after sunscreen.
Wear long-sleeved shirts and long pants
Treat clothing and gear with permethrin

- Permethrin is an insecticide that kills or repels insects like mosquitoes and sand flies.
- Permethrin-treated clothing provides protection after multiple washings.
- Read product information to find out how long the protection will last.
- If treating items yourself, follow the product instructions.
- Do not use permethrin products directly on skin.
- Watch the CDC video How to Use Permethrin .
Keep mosquitoes out of your hotel room or lodging
- Choose a hotel or lodging with air conditioning or window and door screens.
- Use a mosquito net if you are unable to stay in a place with air conditioning or window and door screens or if you are sleeping outside.
Sleep under a mosquito net
- Sleep under a mosquito net if you are outside or when screened rooms are not available. Mosquitoes can live indoors and bite during the day and night.
- Buy a mosquito net at your local outdoor store or online before traveling overseas.
- Choose a mosquito net that is compact, white, rectangular, with 156 holes per square inch, and long enough to tuck under the mattress.
- Permethrin is an insecticide that kills mosquitoes and other insects.
- To determine if you can wash a treated mosquito net, follow the label instructions.
If you are bitten by mosquitoes, avoid scratching the bites and apply over-the-counter anti-itch or antihistamine cream to relieve itching. See Mosquito Bite Symptoms and Treatment .

If you traveled and feel sick, particularly if you have a fever, talk to a healthcare provider and tell them about your travel.
If you need medical care abroad, see Getting Health Care During Travel .
- CDC Yellow Book: Malaria
- Malaria Hotline —770-488-7788 or 770-488-7100
- Malaria Risk Assessment for Travelers
- Choosing the Right Drug to Prevent Malaria
File Formats Help:
- Adobe PDF file
- Microsoft PowerPoint file
- Microsoft Word file
- Microsoft Excel file
- Audio/Video file
- Apple Quicktime file
- RealPlayer file
- Zip Archive file
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.

BRETT A. JOHNSON, MD, AND MONICA G. KALRA, DO
A more recent article on malaria is available.
This is a corrected version of the article that appeared in print.
Am Fam Physician. 2012;85(10):973-977
Patient information : See related handout on prevention of malaria , written by the authors of this article.
Author disclosure: No relevant financial affiliations to disclose.
There are approximately 300 million cases of malaria each year, resulting in 1 million deaths worldwide. Family physicians often encounter patients preparing to travel to malaria-endemic regions. Physicians should have basic knowledge of parasite transmission and malaria prevention. The risk of malaria acquisition is based largely on geographic location and travel season. Most cases occur in sub-Saharan Africa, the Indian subcontinent, and Southeast Asia between the months of May and December. Key elements in prevention include barrier protection and chemoprophylaxis. Travelers to malaria-endemic areas should be advised to use mosquito repellent at all times and bed netting at night. Prophylactic medication should be initiated before travel and continued after return. Travelers should be warned that malaria symptoms can present up to one year after a mosquito bite. Symptoms are vague, and may include fever, chills, arthralgias, and headaches. Travelers experiencing symptoms should seek prompt medical attention.
There are approximately 300 million cases of malaria each year, resulting in 1 million deaths worldwide. 1 Reports from the Centers for Disease Control and Prevention (CDC) indicate that there are between 1,200 and 1,600 cases of malaria annually in the United States. 2 In 2009, there was a 14 percent increase in reported cases of malaria (from 1,298 cases in 2008 to 1,484 cases in 2009). 2 One factor contributing to disease resurgence is global climate change. 3 Between 2011 and 2020, the global mean temperature is expected to rise by 0.4°C. 3 This increase in temperature has been projected to lead to a 30 to 100 percent increase in mosquito abundance worldwide. 3
Most malaria infections in this country occur among persons who have traveled to areas with ongoing malaria transmission. In the United States, cases also can occur through exposure to infected blood products, congenital transmission, or local mosquito-borne transmission. 2
Not only are mosquitoes proliferating with environmental change, but recent findings also suggest that malaria is becoming resistant to treatment. Family physicians can address these issues with a preventive approach that includes traveler education, risk assessment, barrier protection, and chemoprophylaxis.
Sources of Transmission
Five main species of parasites are responsible for transmission of malaria in humans: Plasmodium falciparum , Plasmodium vivax , Plasmodium ovale , Plasmodium knowlesi , and Plasmodium malariae . 4 These protozoa are concentrated in different areas of the world, and each produces a different manifestation of infection. P. falciparum is the most life-threatening form of malaria.
These parasites are transmitted to humans by the bite of an infective female Anopheles mosquito. To produce eggs, the mosquito usually consumes a blood meal, thus needing humans and animals as hosts. The development of the protozoa in the mosquito takes 10 to 21 days, depending on the species of the parasite. After the parasites enter the host's liver, the replication stage begins. Subsequent replication occurs in erythrocytes and may last from one week to one year. Symptoms of malaria appear after the parasites leave the liver and start lysing red blood cells.
Risk Assessment
An individual risk assessment should be conducted for every traveler, taking into account the destination and season of travel. 5 Physicians should provide travelers with resources that discuss risk factors for malaria transmission ( Table 1 ) .
According to the World Health Organization, malaria was endemic in 106 countries in 2010. 6 Most cases occur in sub-Saharan Africa, the Indian subcontinent, and Southeast Asia. A map of worldwide malaria endemicity is available on the CDC Web site at http://cdc.gov/malaria/map/ . Malaria accounts for 5 percent of febrile illnesses in Ethiopia between the months of January and April, and up to 30 percent between the months of May and December. 7
Precipitation is also a contributing factor for vector transmission because riverbeds and stagnant pools of water are breeding grounds for the Anopheles mosquito. Travelers should be advised that the highest risk of malaria is during and after the rainy season. 8
Mosquito Bite Prevention
Mosquito sprays and bed netting are effective in preventing malaria transmission. A trial in the Bolivian Amazon showed that episodes of malaria were reduced by 80 percent among persons using insect repellent and insecticide-treated bed netting. 9
The CDC recommends diethyltoluamide (DEET) and picaridin as repellents for malaria prevention. 10 DEET concentrations between 4 and 30 percent are effective for malaria protection. 11 Higher concentrations are not associated with increased levels of toxicity. The effectiveness of DEET plateaus at a concentration of 30 percent. A formulation of 4 percent offers a complete mean protection time of approximately 90 minutes, whereas a 23 percent formulation offers more than five hours of protection. Adverse effects of DEET include dermatitis, allergic reactions, and rare neurotoxicity. The American Academy of Pediatrics does not recommend DEET for infants younger than two months. 12 The recommendations for DEET use in pregnant and lactating women are similar to those for nonpregnant adults. 11
A 20 percent solution of picaridin is comparable to a 35 percent DEET solution. 13 The highest concentration of picaridin sold in the United States is 15 percent, and the data are insufficient to support adequate protection against Anopheles mosquitoes at this concentration. Picaridin does not cause skin irritation and is safe to use in children and pregnant women.
In 2007, scientists in South America developed a mosquito repellent containing p -menthane-3,8-diol (PMD), a eucalyptus plant extract. 14 The formula is less toxic, cheaper, and more effective against malaria than a 20 percent solution of DEET. 14 In the United States, PMD is available as 65 percent and 10 percent concentrations. 15 The U.S. Environmental Protection Agency recommends these products as repellents against mosquitoes, biting flies, and gnats. 15 Adverse effects include skin and eye irritation. 15
Barriers such as insecticide-treated netting and clothing are as important as repellents in the prevention of malaria. A study in sub-Saharan Africa concluded that bed netting reduces the incidence of malaria by at least 50 percent. 16 Use of clothing treated with permethrin (a synthetic mosquito repellent) is effective in preventing mosquito bites. 17
Chemoprophylaxis
All recommended chemoprophylactic regimens involve taking medication before travel, during travel, and for a period of time after leaving the malaria-endemic region ( Table 2 ) . 18 – 22 Beginning the regimen before travel is necessary to allow the antimalaria agent to enter the bloodstream before exposure to malaria-carrying parasites. 18 Atovaquone/proguanil (Malarone), doxycycline, and mefloquine are the drugs of choice for malaria prevention in most malaria-endemic regions. 18
ATOVAQUONE/PROGUANIL
Atovaquone/proguanil is a good choice for last-minute travelers because it can be started one to two days before travel, as opposed to one to two weeks with some of the other drugs. 18 Common adverse effects include abdominal pain, nausea, vomiting, and elevated alanine transaminase levels. It is contraindicated in patients with a creatinine clearance of less than 30 mL per minute per 1.73 m 2 (0.50 mL per second per m 2 ). 18 Atovaquone/proguanil is a U.S. Food and Drug Administration (FDA) pregnancy category C medication.
DOXYCYCLINE
Doxycycline is taken daily and provides additional protection against many infections, including tick-borne illnesses. 18 Travelers should be aware that photosensitivity may increase in persons with prolonged sun exposure. Other adverse effects include vaginal candidiasis, abdominal pain, and diarrhea. Doxycycline is FDA pregnancy category D, and should be used only if maternal benefits outweigh fetal risks. It is contraindicated in children younger than eight years.
Mefloquine is taken weekly. It is considered safe to use during the second and third trimesters of pregnancy. 18 Resistance to mefloquine is found in areas of China, Myanmar, Laos, Vietnam, and Cambodia. 23 Five percent of patients taking mefloquine will experience neuropsychiatric effects (e.g., insomnia, paranoia, hallucinations, seizures) that lead to discontinuation of the drug. 19 , 20
CHLOROQUINE
Chloroquine (Aralen) was the standard of care for malaria prevention for many years. However, as P. falciparum has become largely resistant to chloroquine, it is now recommended only for travelers going to the Middle East, Central America, Haiti, and the Dominican Republic. 18 Chloroquine can be used in all trimesters of pregnancy and in children of all ages. 18 Adverse effects may include blurry vision, tinnitus, and hearing loss.
Primaquine is used mainly in areas where P. vivax is the primary strain of malaria (e.g., parts of Central and South America). Patients must be tested for glucose-6-phosphate dehydrogenase deficiency before taking primaquine because it may cause hemolysis in affected persons. 21 Other adverse effects include nausea, vomiting, and abdominal pain. 21 Primaquine is an FDA pregnancy category C medication.
Five to 80 percent of patients treated for P. vivax malaria will relapse. 22 As a preemptive measure, patients with P. vivax infection should be treated with a 14-day course of primaquine to prevent further disease. 22 Primaquine therapy should be started on the same day as malaria treatment. 22
Recognition of Illness
Travelers should be warned that adequate chemoprophylaxis does not guarantee full protection against malaria. Symptoms may appear from one week to one year after infection with the parasite. Relapsing illness may occur in patients who have completed a course of treatment. 10 Travelers to malaria-endemic areas should seek medical attention for signs and symptoms of malaria, including fever, chills, headaches, and arthralgias. 10
Presumptive Treatment
Travelers who decline malaria prophylaxis or who will be traveling to remote areas with limited access to health care may be prescribed a three-day supply of presumptive malaria treatment before travel. 23 Travelers should be advised that self-treatment of a possible malaria infection is only a temporary measure, and that prompt medical evaluation is imperative. 23 A three-day course of high-dose oral atovaquone/proguanil or artemether/lumefantrine (Coartem) may be prescribed. 23 Travelers should take the medication if they experience high fevers, chills, or myalgias. 23 Physicians who need assistance with the diagnosis or treatment of malaria should call the CDC Malaria Hotline (855-856-4713).
The Future of Malaria Prevention
A malaria vaccine is being developed for delivery through the World Health Organization's Expanded Programme on Immunization. 24 It is being studied in African infants during the first 13 months of life, and has been reported to reduce transmission of malaria by 65 percent with few adverse effects. 24 Along with barrier protection and chemoprophylaxis, vaccination may eventually play a key role in the eradication of malaria worldwide. 24
Data Sources: We searched PubMed, Essential Evidence Plus, the Cochrane database, and UpToDate using variations of the key term malaria prevention. Search dates: July to September 2010, and July 2011.
Centers for Disease Control and Prevention. Malaria—malaria facts. http://www.cdc.gov/malaria/about/facts.html . Accessed December 12, 2011.
Mali S, Tan KR, Arguin PM Division of Parasitic Diseases and Malaria. Center for Global Health; Centers for Disease Control and Prevention. Malaria surveillance—United States, 2009. MMWR Surveill Summ. 2011;60(3):1-15.
Pascual M, Ahumada JA, Chaves LF, Rodó X, Bouma M. Malaria resurgence in the East African highlands: temperature trends revisited. Proc Natl Acad Sci USA. 2006;103(15):5829-5834.
Freedman DO. Clinical practice. Malaria prevention in short-term travelers. N Engl J Med. 2008;359(6):603-612.
Centers for Disease Control and Prevention. Malaria—disease. http://www.cdc.gov/malaria/about/disease.html . Accessed August 15, 2010.
World Health Organization. World Malaria Report: 2010. http://www.who.int/malaria/world_malaria_report_2010/en/index.html . Accessed December 12, 2011.
Muhe L, Oljira B, Degefu H, Enquesellassie F, Weber MW. Clinical algorithm for malaria during low and high transmission seasons. Arch Dis Child. 1999;81(3):216-220.
Briët OJ, Vounatsou P, Gunawardena DM, Galappaththy GN, Amerasinghe PH. Temporal correlation between malaria and rainfall in Sri Lanka. Malar J. 2008;7:77.
Hill N, Lenglet A, Arnéz AM, Carneiro I. Plant based insect repellent and insecticide treated bed nets to protect against malaria in areas of early evening biting vectors: double blind randomised placebo controlled clinical trial in the Bolivian Amazon. BMJ. 2007;335(7628):1023.
Centers for Disease Control and Prevention. Malaria—malaria and travelers. http://www.cdc.gov/malaria/travelers/index.html . Accessed July 8, 2011.
Fradin MS, Day JF. Comparative efficacy of insect repellents against mosquito bites. N Engl J Med. 2002;347(1):13-18.
American Academy of Pediatrics. Follow safety precautions when using DEET on children. AAP News . 2003;22(5):200-399. http://aapnews.aappublications.org/cgi/content/full/e200399v1 (subscription required). Accessed July 1, 2011.
Frances SP, Waterson DG, Beebe NW, Cooper RD. Field evaluation of repellent formulations containing deet and picaridin against mosquitoes in Northern Territory, Australia. J Med Entomol. 2004;41(3):414-417.
Moore SJ, Darling ST, Sihuincha M, Padilla N, Devine GJ. A low-cost repellent for malaria vectors in the Americas: results of two field trials in Guatemala and Peru. Malar J. 2007;6:101.
U.S. Environmental Protection Agency. Pesticides: regulating pesticides— p -Menthane-3,8-diol (011550) fact sheet. http://www.epa.gov/oppbppd1/biopesticides/ingredients/factsheets/factsheet_011550.htm . Accessed July 1, 2011.
Pennetier C, Corbel V, Boko P, et al. Synergy between repellents and non-pyrethroid insecticides strongly extends the efficacy of treated nets against Anopheles gambiae . Malar J. 2007;6:38.
Kimani EW, Vulule JM, Kuria IW, Mugisha F. Use of insecticide-treated clothes for personal protection against malaria: a community trial. Malar J. 2006;5:63.
Centers for Disease Control and Prevention. Malaria—choosing a drug to prevent malaria. http://www.cdc.gov/malaria/travelers/drugs.html . Accessed August 15, 2010.
Gutman J, Green M, Durand S, et al. Mefloquine pharmacokinetics and mefloquineartesunate effectiveness in Peruvian patients with uncomplicated Plasmodium falciparum malaria. Malar J. 2009;8:58.
Nevin RL, Pietrusiak PP, Caci JB. Prevalence of contraindications to mefloquine use among USA military personnel deployed to Afghanistan. Malar J. 2008;7:30.
Hill DR, Baird JK, Parise ME, Lewis LS, Ryan ET, Magill AJ. Primaquine: report from CDC expert meeting on malaria chemoprophylaxis I. Am J Trop Med Hyg. 2006;75(3):402-415.
Baird JK, Hoffman SL. Primaquine therapy for malaria. Clin Infect Dis. 2004;39(9):1336-1345.
Centers for Disease Control and Prevention. Travelers' health—infectious diseases related to travel: malaria. http://wwwnc.cdc.gov/travel/yellowbook/2012/chapter-3-infectious-diseases-related-to-travel/malaria.htm . Acessed July 8, 2011.
Abdulla S, Oberholzer R, Juma O, et al. Safety and immunogenicity of RTS,S/AS02D malaria vaccine in infants. N Engl J Med. 2008;359(24):2533-2544.
Continue Reading

More in AFP
More in pubmed.
Copyright © 2012 by the American Academy of Family Physicians.
This content is owned by the AAFP. A person viewing it online may make one printout of the material and may use that printout only for his or her personal, non-commercial reference. This material may not otherwise be downloaded, copied, printed, stored, transmitted or reproduced in any medium, whether now known or later invented, except as authorized in writing by the AAFP. See permissions for copyright questions and/or permission requests.
Copyright © 2024 American Academy of Family Physicians. All Rights Reserved.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Ther Adv Infect Dis
- v.8; Jan-Dec 2021

An update on prevention of malaria in travelers
Nelson iván agudelo higuita.
Department of Medicine, Section of Infectious Diseases, University of Oklahoma Health Science Center, 800 Stanton L. Young Blvd., Suite 7300, Oklahoma City, OK 73104, USA
Bryan Pinckney White
Infectious Diseases Clinical Pharmacist, Oklahoma University Medical Center, Oklahoma City, OK, USA
Carlos Franco-Paredes
Department of Medicine, University of Colorado Denver School of Medicine, Aurora, CO, USA
Miranda Ann McGhee
Malaria, a parasitic disease caused by protozoa belonging to the genus Plasmodium , continues to represent a formidable public health challenge. Despite being a preventable disease, cases reported among travelers have continued to increase in recent decades. Protection of travelers against malaria, a potentially life-threatening disease, is of paramount importance, and it is therefore necessary for healthcare professionals to be up to date with the most recent recommendations. The present review provides an update of the existent measures for malaria prevention among travelers.
Introduction
Malaria is a parasitic disease caused by protozoa belonging to the genus Plasmodium . There are four species that exclusively affect humans: Plasmodium falciparum , Plasmodium vivax, Plasmodium ovale, and Plasmodium malariae. Plasmodium species that commonly infect non-human primates can also be responsible for a high proportion of human cases in certain parts of the world as is the case with Plasmodium knowlesi in southeast Asia and Plasmodium simium in Brazil. 1 – 4 All species of malaria are transmitted by the bite of an infective female Anopheles mosquito. Malaria can be also transmitted through blood transfusion, needle sharing, laboratory accidents, organ transplantation, and congenitally from mother to fetus. 5 , 6
Malaria continues to represent a formidable public health challenge. According to the most recent World Malaria Report published in 2020, there were an estimated 229 million cases of malaria worldwide in 2019 with 94% reported from the African region and mostly affecting children younger than 5 years of age. There were an estimated 409,000 deaths globally, with 95% occurring in sub-Saharan Africa. 7 Although there has been progress in reducing the global prevalence and mortality attributable to malaria, the number of cases reported among travelers in the United States (US) has continued to have a stepwise increase of approximately 29.4 cases per year since 1972. 6 A total of 2161 confirmed malaria cases were reported by the Centers for Disease Control and Prevention (CDC) in 2017, a 4% relative increase in confirmed cases compared with 2016 and the highest in 45 years. Most cases originated from West Africa (66.9%) and P. falciparum accounted for the majority of infections (70.5%). Most of the cases of malaria affected travelers who were visiting friends and relatives (VFR traveler) and only about a quarter of US residents with malaria reported taking any form of chemoprophylaxis. There were 27 pregnant women affected, of which 22 were hospitalized. Ten of the pregnant women were US residents, and none took prophylaxis to prevent malaria. 6
Search strategy and selection criteria
We searched PubMed and Google Scholar for articles published up to June 30, 2021 with emphasis in the last 2 decades, using the terms ‘malaria,’ in combination with ‘traveler,’ ‘protection,’ ‘prophylaxis,’ ‘prevention.’ We reviewed these articles, and relevant articles in the references of these articles. Only articles published in English were included.
Clinical presentation
The severity of clinical manifestations due to malaria is primarily determined by previous exposures to Plasmodium spp. and the resulting immune status (i.e. premunition). The degree of parasitemia also plays a significant role in the pathogenetic mechanisms of the infection in the microvasculature. The ability of P. falciparum and, to a lesser degree, P. knowlesi to infect most stages of the lifespan of red cells correlates with higher levels of parasitemia and worse outcomes. Reports of life-threatening malaria caused by P. vivax are increasing in certain areas of the world, with thrombocytopenia being a potential marker of severity that requires further validation. 8 , 9 Most travelers are considered non-immune to malaria and symptomatic disease is therefore seen across all age groups. The incubation period is of approximately 2 weeks for malaria caused by P. falciparum , P. knowlesi , and P. vivax . For P. ovale and P. malariae , the incubation period is of about 2–3 weeks and 18–35 days, respectively. 10 – 13 Temperate climate P. vivax ( P. vivax var hibernans ) was of great importance until the middle of last century and was characterized by long incubation periods of up to 8–10 months. Temperate climate P. vivax malaria is still endemic in the Korean peninsula. 14 – 16
The incubation period can also be altered (i.e. prolonged) by agents used for chemoprophylaxis and by antibiotics that are not commonly used to prevent or treat malaria such as rifampin, azithromycin, and ciprofloxacin. 17 – 19 Malaria can present as early as 1 week after the initial exposure and as late as several years after leaving a malaria zone irrespective of chemoprophylaxis use. This is especially true with P. vivax or P. ovale when no liver stage schizonticide is taken as part of prophylaxis. 13 , 18 , 19
The presentation of malaria can be vague and nonspecific. Fever, malaise, headache, chills, and sweats are common but gastrointestinal and respiratory complaints may predominate. Fever in a returning traveler should be considered a medical emergency and expedited evaluation for life-threatening infections is mandatory. Failure to consider the diagnosis of malaria is not infrequent and the diagnosis can be difficult, as fever is not always present during the initial evaluation. If the first blood films are negative, both thick and thin blood films or rapid diagnostics should be repeated twice 8–24 h apart. 13 , 19
There are no pathognomonic physical exam findings for malaria but several variables could be used to predict a higher likelihood of the disease. Splenomegaly, fever, a white blood cell count <10,000 cells/l, platelet count <150,000/μl, hemoglobin <12 g/dl, eosinophils <5%, and hyperbilirubinemia have been associated with parasitemia. 20 , 21 Malaria is a notifiable disease in every state of the United States.
Educating the traveler
The risk of acquiring malaria is determined by a variety of factors inherent to the traveler, itinerary, and the geographic area being visited. The risk assessment should be therefore individualized and ideally occur at least 4–6 weeks before departure.
Malaria is endemic in 90 countries and territories ( Figure 1 ) and its transmission is usually continuous throughout the year in tropical regions and seasonal in temperate zones of the world. The intensity and extent of transmission within a country varies and may be focal. 7 , 22 There are several resources with country-specific data regarding malaria transmission to help clinicians with decision making. It is important to note that despite the great value offered by these resources, reliable data on area-specific risks within a predetermined region/country is difficult to predict 23 and that several professional organizations publish recommendations that differ, sometimes to a great extent. In general, studies have shown that the highest risk of malaria transmission occurs in sub-Saharan Africa and Papua New Guinea. 6 , 24 – 27 Chemoprophylaxis should be therefore prescribed routinely to travelers visiting these regions, Pakistan and India, regardless of whether they will be visiting an urban or rural setting. It is important to note that most, but not all, urban and tourist destinations in southeast Asia, Central and South America do not have sufficient risk of malaria transmission to warrant routine prophylaxis. 27 , 28

Malaria-endemic countries.
Certain populations are at higher risk of acquiring the disease or suffering from its complications. Immigrants who have settled in developed countries and return to their home countries as VFR travelers are highly vulnerable. 6 , 23 , 26 Some of the factors that affect a VFR’s risk of illness include beliefs that they are immune to diseases that they might have acquired during childhood (e.g. malaria), access and trust of the healthcare (e.g. asylum seekers and new immigrants), lack of awareness of the risks associated with travel, cost, and language barriers among others. 29 , 30 Pregnant women are at high risk of developing potentially fatal complications related to malaria. 31 Women who are pregnant or who are likely to become pregnant should be advised against travel to malaria-endemic zones, as there is no chemoprophylactic regimen that is completely effective (see section 8.2 for more details).
There are many other aspects that need to be considered when determining the risk of acquisition of malaria. These include the length and season of the trip, rural versus urban setting, altitude of the destination, accommodation characteristics, outdoor exposure during night-time hours in locations with considerable exposure to mosquitoes, and adherence to mosquito avoidance precautions and chemoprophylaxis. 23 Travel health practitioners need to inform travelers of the life-threatening nature of malaria infection and the importance of prevention.
Personal protection measures
Anopheles spp. feed mainly between dawn and dusk, and measures to decrease exposure during this time is of paramount importance. Contact with mosquitoes can be reduced by the use of screened accommodations and mosquito bed nets (preferably insecticide impregnated), appropriate application of repellents, and wearing clothes that cover most of the body. 23 , 32 – 34
A wide array of popular devices designed to repel mosquitoes are available on the market. A few examples include coils, candles, and heat-generating devices. The effectiveness of these devices is not supported by robust studies and should therefore be used with the addition of proven measures. 34
In general, topical repellents can be divided into synthetic chemicals and plant-derived essential oils. 35 , 36 The best-known chemical insect repellents are N, N -diethyl- m -toluamide, also known as N, N -diethyl-3-methylbenzamide (DEET), and picaridin. Oil of lemon eucalyptus (OLE) or PMD (chemical name: para-mentahne-3,8 diol), IR3535 [chemical name: 3-( N -butyl- N -acetyl)-aminopropionic acid, ethyl ester], and 2-undecanone are considered either derivatives or synthetic products of natural materials. 33 , 34
The efficacy and duration of protection are subject to factors such as ambient temperature and humidity, degree of perspiration and level of activity, exposure to water and other variables. The degree of protection to a determined species of mosquito or tick varies according to the active ingredient and the duration of protection is proportional to the concentration of the product. 33 , 34 For DEET, picaridin, and IR3535, a concentration of at least 20% and for PMD 30% is recommended. Most repellents can be used on children aged >2 months with the exception of OLE, which should not be used on children aged <3 years. No additional precautions for using registered repellents on children or pregnant or lactating women are otherwise needed. 33 , 34 It is also important to remember that DEET can have an unpleasant odor for some and can dissolve plastic. In addition, the sunscreen and repellent should be used as separate products and the sunscreen should be applied first.
The Environmental Protection Agency reviews and approves repellents based on efficacy and human safety. A repellency awareness graphic is available on the labels of insect repellents. More information is available on the following website: www.epa.gov/insect-repellents/repellency-awareness-graphic .
Chemoprophylactic agents
Please refer to Table 1 for doses and schemes used for the different drugs.
Chemoprophylactic agents in malaria.
CrCl, creatinine clearance; G6PD, glucose-6-phosphate dehydrogenase.
Atovaquone/proguanil
Atovaquone inhibits the parasitic electron transport. Proguanil is metabolized through CYP2C19 to cycloguanil, which acts a parasitic dihydrofolate reductase inhibitor. 37 The drug’s synergistic effect is caused by proguanil’s ability to increase atovaquone activity to collapse the mitochondrial membrane potential. 38 Atovaquone is poorly absorbed, with bioavailability reaching 23% when taken with food. It is extensively protein bound (>99%) and is primarily (94%) hepatically eliminated with limited metabolism. It has a half-life of 55.9 h with multiple doses. 37 Proguanil is extensively absorbed regardless of food with a bioavailability of 60–75%. Approximately 40–60% of proguanil is excreted renally with a half-life of 12–21 h. 38
Studies have shown a 96–100% protection efficacy against P. falciparum . The medication is well tolerated with the most common side effects reported being abdominal pain, nausea, vomiting, and headache. Abbreviated regimens have been examined in an attempt to improve compliance. The doses used for the treatment of malaria (four tablets daily for 3 consecutive days) provides protection against malaria for >4 weeks. A study done in Australia examined the efficacy of this regimen in adults traveling to malaria-endemic areas with low-to-medium risk for up to 4 weeks. Most participants complied with the regimen, although 43.3% reported side effects. No traveler developed malaria, although the study was not designed and did not have the statistical power to determine effectiveness. 39 An observational study designed to detect prophylactic failure with a twice-weekly regimen of atovaquone/proguanil among long-term expatriates (⩾6 months) in West Africa found no cases of malaria. In comparison, the malaria rates were 11.7/1000 person-months in the group taking no prophylaxis, and 2.06/1000 person-months in the group taking weekly mefloquine. 40 Other investigators have examined and concluded that discontinuation of the medication 1 day after return as opposed to the 7 days did not alter efficacy. 41 Despite these promising results, a modified regimen of atovaquone/proguanil needs to be supported by more robust data from clinical trials with a larger sample size and higher-risk destinations. 42
Doxycycline
Doxycycline inhibits protein synthesis by binding to the 30S ribosomal subunit. Doxycycline may also inhibit dihydroorotate dehydrogenase, a mitochondrial enzyme involved in pyrimidine synthesis. 43 Doxycycline is almost completely absorbed in the duodenum with a bioavailability of 95%. Food decreases absorption by 20%. The volume of distribution is 0.7 l/kg with protein binding of 82–93%. It achieves high concentrations in the liver and it is excreted unchanged with 35–60% in the urine and the rest in feces. 44
Doxycycline has an efficacy between 92% and 96% for P. falciparum and 98% for primary P. vivax infection. The most common side effects are photosensitivity, nausea, vomiting, and an increased frequency of vaginal yeast infections. 45 The medication should be taken with at least 8 oz of water while in an upright position and not just before going to bed, to avoid esophagitis. Doxycycline monohydrate or enteric-coated doxycycline hyclate are better tolerated than generic doxycycline hyclate. It is also common practice to prescribe 150 mg of fluconazole for women that intend to use the medication as a standby treatment for vaginal candidiasis. There are insufficient data to make recommendations regarding the interchangeability between doxycycline and minocycline for this indication. Minocycline should be stopped 1–2 days before travel for those taking it chronically and be replaced with doxycycline. The minocycline can be resumed once the course of doxycycline has been completed. 45
The exact mechanism of action is unknown but protein synthesis inhibition has been hypothesized. 46 Mefloquine is well absorbed with a bioavailability of 87–89%. It has high protein binding of 98%, with a mean volume of distribution of 22 l. 47 Mefloquine is metabolized by CYP3A4 into carboxymefloquine, an inactive metabolite. 48 Its terminal half-life is 14–21 days with primarily bile and feces elimination. 47
Controversy has surrounded the use of this agent due to its safety profile. 49 , 50 Mefloquine has been associated with a lengthy list of side effects including gastrointestinal discomfort, headache, and dizziness. More severe neuropsychiatric side effects such as visual disturbances, severe depression leading to suicide, sensory and motor neuropathies, memory deficits, hallucinations, aggression, seizures, psychosis, irreversible vertigo, and encephalopathy have been reported. The US Food and Drug Administration (FDA) issued a black-box warning in 2013 about the risk of neuropsychiatric side effects. In the US, any traveler receiving a prescription for mefloquine must also receive a copy of the FDA medication guide, which can be found at https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/019591s023lbl.pdf . 51 The European Medicines Agency updated the product information and mandated that all European Union members ensure that healthcare professionals are aware and communicate to their patients the risk neuropsychiatric and other adverse events. In addition, it was stipulated that only travelers without a contraindication to the medication receive a prescription and that the traveler carry an alert card at all times. 52
The risk of developing severe or disabling neuropsychiatric side effects ranges from 1/607 to 1/20,000 compared with a rate for chloroquine of 1/1181 to 1/13,600. 50 Mefloquine is contraindicated in the setting of allergy to the medication or related compounds (e.g. quinine) or any excipient. Active or recent history of depression, generalized anxiety disorder, psychosis, schizophrenia, convulsions, cardiac conduction abnormalities and treatment with halofantrine or ketoconazole are also contraindications. 52
Mefloquine is an ideal chemoprophylactic agent for long-term travelers, children, and pregnant women, but due to the potential toxicities, it should be reserved when other agents are contraindicated and for areas with high malaria risk such as sub-Saharan African and parts of Oceania.
Chloroquine and hydroxychloroquine
Chloroquine and hydroxychloroquine’s exact mechanism of action is unknown. They are thought to concentrate in lysosomes and interfere with parasitic processing of hemoglobin. 53 Chloroquine has a bioavailability of 89%. Hydroxychloroquine has a bioavailability of 74%. The pharmacokinetics of these drugs are complex, with a large volume of distribution (greater than 50,000 l has been reported), three-compartment pharmacokinetics, and reported half-lives of over 100 days. They both concentrate in the liver. Approximately 23–38% is excreted unchanged in the urine, 17–18% is excreted as metabolites in the urine, with the remainder being excreted in feces or stored long term in lean tissues. 54
The medication is well tolerated with the most common reported side effects being gastrointestinal disturbances, dizziness, blurred vision, insomnia, and pruritus. The medication can exacerbate psoriasis, and although rare in the doses used for prophylaxis, retinopathy can occur. The medication is safe in pregnancy. 51
The exact mechanism of action of primaquine is unknown. Possible mechanisms are impeding mitochondrial metabolism or oxidative stress. It is rapidly absorbed with a plasma peak in 1–3 h and a volume of distribution of 3 l/kg. It accumulates in the brain, liver, skeletal muscle, lungs, and heart. Primaquine is metabolized into carboxyprimaquine and other metabolites, by oxidases and dehydrogenases. Less than 5% is eliminated unchanged in the urine. 55
Primaquine phosphate has two roles in prevention. It can be used as a causal prophylactic agent against all Plasmodium spp . and for presumptive anti-relapse therapy (PART), also known as terminal prophylaxis, for P. vivax and P. ovale . The dosing should overlap with the blood schizonticide and therefore when chloroquine, doxycycline, or mefloquine are used for primary prophylaxis, primaquine is usually taken during the last 2 weeks of postexposure prophylaxis. When atovaquone–proguanil is used for prophylaxis, primaquine may be taken during the final 7 days of atovaquone–proguanil, and then for an additional 7 days. Primaquine should be given concurrently with the primary prophylaxis medication. However, if that is not feasible, the primaquine course in the form of terminal prophylaxis should still be administered after the primary prophylaxis medication has been completed. 56 Terminal prophylaxis with primaquine (or tafenoquine, see below) is particularly important for long-term travelers returning from highly endemic areas of P. vivax transmission in the Pacific Islands (i.e. Papua New Guinea, Vanuatu, and Solomon Islands) or among those returning from countries that constitute the Horn of Africa.
The efficacy for prophylaxis is considered to be over 85% against P. falciparum and P. vivax and around 95% when used for PART. The most common side effects are abdominal pain, nausea, and vomiting. Severe hemolysis in persons with glucose-6-phosphate-dehydrogenase (G6PD) deficiency and methemoglobinemia can occur. The medication is contraindicated in the setting of G6PD deficiency, nicotinamide dehydrogenase methemoglobin reductase deficiency, pregnancy, known hypersensitivity to primaquine, and illnesses that manifest with tendency for granulocytopenia. A G6PD testing should be performed before use of the medication. 56
Tafenoquine
Tafenoquine’s exact mechanism of action is unknown. Mitochondrial dysfunction leads to Plasmodium spp. death. Absorption is slow and increased with a high-fat meal. It is extensively (>99.5%) protein bound with a volume of distribution of 1600 l. The terminal half-life is 15 days. It is thought to be excreted unchanged, but complete information on excretion is unknown. 57
Tafenoquine succinate is formulated as either a 100 mg or 150 mg tablet. The medication has been approved by the FDA and in Australia for the primary prevention of malaria for persons aged ⩾18 years and for the radical cure of P. vivax in persons older than 16 years of age. 57 – 59 It is important to note that the medication is approved in two separate formulations from two different manufacturers. The 60° Pharmaceuticals manufacture Arakoda ® and Kodatef ® for causal prophylaxis in the US and Australia, respectively. In a partnership with Medicines for Malaria Venture, GlaxoSmithKline (GSK) manufactures Krintafel ® and Kozenis ® for radical cure of P. vivax , also in the US and Australia, respectively. Tafenoquine is also licensed for PART. For P. ovale , tafenoquine can be used off label for radical cure. 60 – 62
Tafenoquine has only been studied for radical cure of P. vivax malaria when used with chloroquine, and the medication should therefore be co-administered with chloroquine only. CDC continues to recommend the off-label use of tafenoquine for radical cure of P. ovale and like with P. vivax , it should be co-administered with chloroquine only.
Tafenoquine has a half-life of approximately 2 weeks allowing weekly administration for primary prophylaxis after a loading dose and one dose when used for radical cure. When used for primary prophylaxis, the medication is taken at a dose of 200 mg daily for 3 days prior to travel, weekly during travel, and then once after return. The last dose should be taken 7 days after the last maintenance dose while in the malaria-endemic area. For the radical cure of P. vivax , the dose is 300 mg once. 57 , 58 The efficacy of the medication for causal prophylaxis varies between 86% and 100%. The malaria recurrence-free rate at 6 months ranges from 62% to 89%. 58
The medication is contraindicated in G6PD-deficient individuals. Quantitative G6PD testing (rather than qualitative, which is usually appropriate for primaquine use) is required, which might logistically be difficult to accomplish for last-minute travelers. 63 , 64 The medication is contraindicated in pregnancy and in breastfeeding women if the infant has G6PD deficiency or if the infant’s G6PD status is unknown. Its safety in children has not been established. 58
Malaria standby emergency treatment (SBET)
The concept describes the self-administration of emergent malaria treatment brought from the country of origin for use when no medical attention is available or for use after the diagnosis has been established. The topic is controversial and the appropriate setting for its use varies according to different national guidelines. 65 There is great variation in the proportion of travelers that appropriately use this strategy as response to the development of fever which is of primordial importance given the life-threatening nature of the disease. 66
For travelers taking chemoprophylaxis and for whom SBET is prescribed, the drug that is being used for chemoprophylaxis should not be used for treatment. Once treatment is completed, chemoprophylaxis should be resumed. If atovaquone/proguanil is being used, it can be resumed immediately after treatment. If another agent is being used, it can be resumed 1 week after completion of treatment. Atovaquone/proguanil and artemether/lumefantrine, two regimens approved in the US, can be used for SBET. Artemether/lumefantrine can be used during pregnancy. 67 , 68 The medications should be bought and taken from the country of origin, given the high rates of counterfeit in malaria-endemic countries. 51 Mefloquine should be avoided for this indication given the potential toxicity. In addition, artemether/lumefantrine should not be used for SBET if mefloquine is being used for chemoprophylaxis. 51 Other regimens such as the combination of doxycycline and quinine require multiple doses which are frequently associated with side effects.
SBET alone can be considered if traveling to low malaria transmission areas such as most of southeast Asia and South America. If possible, SBET should only be considered when traveling to remote areas where medical attention is hampered by a lack of medical services, or quality medications and access to a medical evaluation is not readily available within 24 h. 65 It is important to emphasize to the traveler that the development of fever requires immediate evaluation regardless of SBET use.
Vaccines have not been approved for the prevention of malaria among travelers. The RTS,S/AS01 vaccine has been studied in two phase III trials in Africa with ages ranging from 5–17 months. 69 – 71 The largest malaria vaccine trial done in Africa included 15,459 infants and young children and showed that the vaccine prevented 39% of cases of malaria and 29% of cases of severe malaria over a 4-year follow-up period. For those that received four doses, 1774 cases of malaria were prevented for every 1000 children vaccinated. Efficacy waned with time. 69 A recommendation to include the vaccine in the national immunization programs has not been made, and a pilot study is currently underway in Ghana, Kenya, and Malawi. 72 Although vaccines can have a great impact in achieving the goals of malaria eradication, further research is needed to be considered ideal for travelers. 73
Choosing an antimalarial agent for chemoprophylaxis
The choice of a chemoprophylactic agent ( Tables 2 2 – 4 ) requires consideration of several factors such as the travel destination, layovers, season, length of travel, traveler’s health, potential side effects and medication interactions, preference, and cost.
Cost of malaria chemoprophylaxis for adults for one week of travel, including required pre- and post-travel dosing. Prices obtained from GoodRx ( https://www.goodrx.com/ ).
US, United States.
Advantages and disadvantages of malaria chemoprophylaxis.
G6PD, glucose-6-phosphate dehydrogenase; US, United States.
Drug interactions with malaria chemoprophylaxis agents. 74
B-blockers, beta blockers; US, United States.
P. falciparum has developed resistance to all antimalarials, and knowledge about its geographic distribution is important in decision making. 51 , 75 Resistance to chloroquine was first observed in southeast Asia and South America in the 1950s and subsequently spread to most parts of the world excluding the Caribbean and Central America, west of the Panama Canal. 76 Resistance is the result of a point mutation in the PfCRT protein that localizes to the digestive vacuole of the Plasmodium spp. This results in the inability of chloroquine to concentrate within the digestive vacuole and form complexes with toxic heme moieties that interfere with detoxification mechanisms. 77 – 80
Resistance of P. vivax to chloroquine was documented in 1989 when Australians repatriated from Papua New Guinea failed routine treatment. 81 Subsequent reports from Indonesia, Myanmar, and India corroborated findings. 82 The cause of resistance in P. vivax remains elusive due to the nature of the parasite’s dormant phase in the liver, low parasitemia, and difficulty in distinguishing relapse, recrudescence, and reinfection. 83
Special populations
Infants, children, and adolescents.
Prevention recommendations for malaria in children are similar to that in adults and include assessment of risk based on travel itinerary, education of mosquito avoidance, determination of chemoprophylaxis, and educating parents to seek early medical care if fever develops during or soon after travel. 84 All chemoprophylactic agents used in adults, with the exception of tafenoquine, are available for children. There are several observations to remember: atovaquone/proguanil should not be administered to children weighing less than 5 kg and doxycycline in those younger than 8 years of age. The product’s label should be carefully followed for pediatric dosing of malarial chemoprophylaxis. Due to difficulty in pediatric dosing, pulverizing tablets into specified dosing can be done, with the assistance of a pharmacist. Due to bitter taste, medications can be mixed into or crushed into food. Specific contraindication for use of repellents in children was described earlier in this document.
Pregnancy and breastfeeding
Pregnant women are particularly susceptible to malaria infection due to immunologic changes that occur during pregnancy. P. falciparum can concentrate in the placenta and lead to miscarriage, stillbirth, preterm birth, and low birth weight. 85 – 87 Congenital infection with detectable parasitemia from 24 h to 7 days of life is another important complication. 88 – 90
Pregnant women should be advised not to travel, if at all possible, as no chemoprophylactic regimen is 100% effective. For women that chose to travel to a malaria-endemic region, emphasis on mosquito avoidance measures and chemoprophylaxis should be provided. In the US, malaria chemoprophylaxis in pregnancy is limited to mefloquine and chloroquine.
In some countries, atovaquone/proguanil is used for either treatment or prophylaxis of malaria during pregnancy. Although the available safety data for its use in pregnancy are reassuring, well-established trials are needed before a definite recommendation can be made. 91 , 92 Doxycycline is also used during the first trimester in several European countries if there are compelling reasons for chemoprophylaxis and if no alternative is available. It is important to remember that doxycycline needs to be administered for 4 weeks after return from a malaria-endemic region. 93
In all women of childbearing age, plans for conception during travel should be discussed. All breastfeeding mothers should be counseled that maternal use of chemoprophylaxis does not provide protection to a breastfed infant, as only a limited amount of medication is secreted in the breastmilk. All chemoprophylactic agents can be administered to breastfeeding mothers except primaquine and tafenoquine, unless G6PD deficiency has been excluded in both the mother and newborn.
Immunosuppressed travelers
Immunosuppressed patients, including those with human immunodeficiency virus (HIV), are more likely to develop severe malaria. 22 Immunosuppressed travelers should be provided chemoprophylaxis similarly to those who are immunocompetent, with additional consideration of the possibility of drug interactions.
Interactions with malaria prophylaxis are primarily seen with cancer-related therapies, anti-rejection medications used after transplantation, and medications used to treat HIV ( Table 4 ). Drug interactions related to anti-retrovirals can be checked on the University of Liverpool HIV Drug Interactions website ( https://www.hiv-druginteractions.org/ ). Typical cancer-directed therapies that can result in interactions include the use of tamoxifen with chloroquine that results in an increased risk of retinopathy, increased levels of platinum-based chemotherapies with tafenoquine, and increased levels of methotrexate when used with doxycycline. In those who have undergone transplantation, chloroquine can increase cyclosporine levels. In travelers with HIV, interactions occur primarily with protease-inhibitor-based regimens and with some non-nucleoside reverse-transcriptase inhibitors such as efavirenz, nevirapine, and etravirine. 74 , 94

Long-term travelers
A long-term traveler is a person visiting a malaria-endemic region for longer than 6 months. Long-term travelers represent a high-risk group, as they tend to underuse personal protective equipment and adhere poorly to anti-malaria prophylaxis. 32
Chloroquine has a good safety record for the treatment of rheumatologic conditions, suggesting it can be used for long-term malaria prophylaxis. For long-term use (>5 years) of chloroquine, a baseline eye examination with bi-annual follow up is recommended to screen for potential retinal toxicity. 51 Long-term mefloquine use is shown to be safe. Tolerability has been variable and related to neuropsychiatric events that usually occur early in the course of prophylaxis. 32 , 95 , 96 Atovaquone/proguanil has been used up to a duration of 34 weeks with good tolerability, with diarrhea being the primary side effect. 97
The tolerability of doxycycline was evaluated in a study of 600 military personnel in Cambodia for 1 year and 900 men deployed to Somalia for 4 months. The medication was well tolerated, with gastrointestinal events and photosensitivity being the most common reported side effects. 98 Another study with 228 US Peace Corps volunteers who took doxycycline prophylaxis for an average of 19 months showed a similar side-effect profile. 99 Doxycycline is approved by the FDA for a duration of 4 months. The use of primaquine for up to 52 weeks is safe with mild non-clinically significant methemoglobinemia being the most common side effect. 100
Relapse prevention
PART is an intervention used to eradicate the quiescent liver hypnozoites of P. vivax or P. ovale with medications such as primaquine and tafenoquine. Primaquine is FDA approved at a dose of 0.25 mg/kg (15 mg base) daily for 14 days. 51 The World Health Organization guidelines recommend a higher dose of primaquine (0.5 mg/kg or 30 mg base daily for 14 days) for strains of P. vivax acquired in East Asia and Oceania, 101 and CDC recommends the higher dose regardless of geographic site of acquisition. 51 Tafenoquine should be administered as a single 300 mg dose and it should ideally overlap with blood-stage treatment or the last dose of prophylaxis. If this is not feasible, tafenoquine may be taken as soon as possible afterward. 62
The use of PART is appropriate for travelers that have visited P. vivax -endemic areas, even if P. falciparum is present, and especially for prolonged stays. 50 It is important to remember that if either primaquine or tafenoquine are used as prophylactic agents, PART is not needed.
There are several important caveats with the use primaquine. Adherence to a 14-day course is poor, and life-threatening hemolytic reactions may occur if G6PD deficiency is not recognized. To address the first limitation, two randomized controlled trials in G6PD-normal patients compared a shorter primaquine regimen consisting of 1 mg/kg day for 7 days with high-dose primaquine for 14 days. Both studies showed no difference in efficacy between the 7-day and 14-day course. However, there were more side effects reported in the 7-day arm in one of the studies. 102 , 103
Blood donation
Transfusion-transmitted malaria was first described in the turn of last century and is an important form of transmission in malaria-endemic areas. 104 A recent review concluded that the median worldwide prevalence of malaria parasitemia in healthy blood donors was 10.54% by microscopy, 5.36% by molecular techniques, and 0.38% by rapid diagnostic tests. 105
Storage of blood under refrigeration is deleterious to Plasmodium spp. Refrigeration at 4°C decreases parasitemia rapidly, nonetheless Plasmodium falciparum has been shown to survive in stored whole blood or plasma at this temperature for approximately 18 days and can remain microscopically detectable for up to 28 days when frozen but with diminished infectivity. 106
The incubation period of transfusion-transmitted malaria is longer than mosquito-transmitted malaria which could lead to a lack of suspicion and delay in the diagnosis of the disease; especially in non-endemic regions. 104 A study that examined transfusion-transmitted malaria in the US between 1963 and 1999 found that the median incubation period was 10 days but ranged from 1 to 180 days. 107
As of April of 2020, the FDA recommends that non-resident travelers of an endemic country or those who are residents of an endemic country but have lived in a non-endemic region for more than 3 consecutive years and are returning from malaria-endemic areas defer blood donation for 3 months after arrival as opposed to 1 year as was previously recommended. This group does not necessarily need to defer donation, as pathogen reduction techniques could be used and allow the collection of components from otherwise-eligible donors. For former residents of malaria-endemic regions that have lived in a non-endemic region for less than 3 consecutive years and for people that have been diagnosed with malaria, donation of blood products should be deferred for 3 years, and this recommendation remains unaltered in the most recent update. 108
The prevention of malaria in travelers continues to be challenging. A multitude of factors determine the risk of malaria acquisition among travelers. The knowledge of such factors and the available preventive measures are of vital importance in being able to provide evidence-based recommendations to travelers. This knowledge should not be restricted to specialized travel medicine professionals as a significant percentage of travelers seek attention from general practitioners. It is therefore imperative for practitioners to be familiar with the most recent guidance and available resources (e.g. internet based, specialized travel clinics available in the community, etc.) to be able to provide safe, effective, and affordable care to the traveler.
Authors contributions: Conceptualization, retrieval of articles for review, critical revision of original draft and approval of final manuscript: Nelson I. Agudelo Higuita, Miranda McGhee, Bryan White, Carlos Franco-Paredes
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.

Contributor Information
Nelson Iván Agudelo Higuita, Department of Medicine, Section of Infectious Diseases, University of Oklahoma Health Science Center, 800 Stanton L. Young Blvd., Suite 7300, Oklahoma City, OK 73104, USA.
Bryan Pinckney White, Infectious Diseases Clinical Pharmacist, Oklahoma University Medical Center, Oklahoma City, OK, USA.
Carlos Franco-Paredes, Department of Medicine, University of Colorado Denver School of Medicine, Aurora, CO, USA.
Miranda Ann McGhee, Department of Medicine, Section of Infectious Diseases, University of Oklahoma Health Science Center, 800 Stanton L. Young Blvd., Suite 7300, Oklahoma City, OK 73104, USA.
- Português Br
- Journalist Pass
Mayo Clinic Q and A: Malaria continues to be a significant travel-related disease
Liza Torborg
Share this:

ANSWER: While there is no vaccine for malaria , it is essential that you receive medication to prevent malaria before you go to Tanzania. Although cases of malaria worldwide are decreasing, malaria continues to be a significant travel-related disease that carries a risk of serious illness and death. To discuss the specific malaria medication you need and other travel-related health precautions you should take, consult with a travel medicine expert well before your trip.
Malaria is a disease caused by a parasite that is transmitted to humans through the bites of infected mosquitoes. People who have malaria usually get a high fever, headache and shaking chills. Malaria symptoms typically begin within a few weeks after being bitten by an infected mosquito. Malaria can be fatal, particularly when caused by the variety of parasite called Plasmodium falciparum that’s common in tropical parts of Africa. The risk of death increases in people who have not been exposed to it previously.
Travel to certain parts of the world poses a higher risk of malaria due to the presence of the more potent type of malaria there, coupled with a higher density of mosquitoes within those areas. Its risk is highest for those traveling to countries in sub-Saharan Africa, followed by developing countries in Oceania. In the Western hemisphere, malaria risk is highest in Haiti and the Dominican Republic. There is also a risk in many countries of Southeast Asia, Central America and South America. The number of people returning to the U.S. with malaria has been increasing in past decades, and most cases have been Plasmodium falciparum .
The medications used to prevent malaria are very effective. It’s important that you get the correct type of medication for the area where you are traveling, though, and carefully follow the directions on how to take it. Different parts of the world have different species of malaria and require different medications for prevention. U.S. travelers who take preventive medication and still get malaria or die from the disease are those who take the wrong medication for their region of travel or take the medication incorrectly.
Those planning to travel to countries that have a risk of malaria should talk with their health care provider, make an appointment with their local travel clinic or visit the Centers for Disease Control and Prevention website to find the best preventive medication to take for their region of travel.
In addition to taking the right medication, follow other precautions to decrease your risk during your trip. That includes wearing long sleeves and pants while in these areas and applying insect repellent to exposed skin.
Insect repellents that are most effective contain DEET, icaridin or lemon eucalyptus extract. Apply repellent to your exposed skin in the morning after you’ve put on sunscreen. Reapply it in the early evening. If you don’t have netting for your bed, add a third layer of repellent before going to bed. For additional protection, use the insecticide permethrin on your clothing.
If you notice symptoms of illness after your trip, see a health care provider right away. Tell your provider of your recent travels. Effective treatment for malaria is available in the U.S. Because malaria is so uncommon in this country, however, providers may not be familiar with diagnosing or treating it, and malaria could be misdiagnosed as a viral illness. Treatment for malaria requires infectious disease expertise, and an infectious disease specialist should be consulted whenever malaria is suspected. Treatment for malaria involves IV or oral medications, depending on the severity of the illness. — Dr. Abinash Virk , Infectious Diseases, Mayo Clinic, Rochester, Minnesota
****************************
Related Articles
- Pretty Parasites With Dr. Pritt, part 5: Bad-news bugs published 11/2/18
- Mayo Clinic Minute: Tips for traveling to developing countries published 8/4/17
- Infectious Diseases A-Z: Traveling abroad? You may need to be vaccinated published 5/22/17
- Less Is More: Losing Weight, Gaining Perspective Heart procedure for AFib better than drug therapy for reducing episodes

- Quick Links
- Make An Appointment
- Our Services
- Price Estimate
- Price Transparency
- Pay Your Bill
- Patient Experience
- Careers at UH
Schedule an appointment today

Malaria Vaccine for Travel: Protect Yourself and Your Family Against Malaria When Traveling Abroad
What is malaria.
Malaria is a serious and sometimes fatal blood infection that is very common in tropical and semitropical parts of the world, including south Asia, parts of the Caribbean, Central America and equatorial South America. Transmission in the United States is exceptionally rare – almost all cases are found in travelers and immigrants returning from countries where malaria is prevalent.
How is Malaria Spread?
Malaria is caused by a parasite that is commonly carried by a certain type of mosquito. The parasite enters the human bloodstream following a mosquito bite. Therefore, it is vital that, in addition to taking a pre-travel regimen of medications, travelers take precautions to protect themselves from mosquito-borne illnesses .
Although mosquitoes are the most common mode of transmission, malaria can also be transmitted in many of the same ways as other blood-borne illnesses, including organ transplants, blood transfusions, and shared needles and syringes.
Symptoms of Malaria
Symptoms may present weeks or even months after being infected and may include:
- High fever (greater than 102°F)
- Flu-like symptoms including chills, headache and muscle aches
A Medical Regimen Can Protect You and Your Family from Malaria
Although malaria can be a deadly disease, illness, and death from malaria can usually be prevented with a regimen of pre-travel (4-6 weeks before departure) medications in the form of pills. Because malaria symptoms may not appear for some time, your doctor may advise you to continue taking malaria vaccine medications for up to four weeks post-travel.
A travel malaria medication regimen is recommended for those traveling to India and most African nations, including but not limited to:
- South Africa
Please speak to the travel medicine specialists at the UH Roe Green Center for Travel Medicine & Global Health for more information about recommended vaccines and disease prevention.
Malaria Protection for U.S. Travelers
Valerie Williams, RPh Howard University School of Pharmacy Washington, DC
US Pharm. 2023;48(12):38-43.
ABSTRACT: In humans, malaria is caused by intraerythrocytic protozoa of the Plasmodium genus. These parasites are transmitted through the bite of an infected female mosquito of the Anopheles species, such as Plasmodium falciparum and Plasmodium vivax . Most malaria infections in the United States occur in persons who have traveled to regions with ongoing malaria transmission. Prevention of mosquito bites is a fundamental goal. Malaria chemoprophylaxis depends on individual criteria determined by clinical and laboratory examinations as well as travel conditions (destination, season, duration of stay, and local living conditions). Standby medication should be prescribed for self-treatment of fever in areas where medical care is not readily available. Malaria is curable, but symptoms can become more severe if untreated, and the disease can eventually be fatal.
Malaria is a preventable and curable infectious disease that occurs in certain countries, mainly in rural areas, and requires the presence of standing water. In humans, malaria is caused by intraerythrocytic protozoa of the Plasmodium genus. Malaria parasites are transmitted—usually in the evening or at night—through the bite of an infected female mosquito from species of the Anopheles genus. 1 Malaria can also be transmitted congenitally from mother to fetus or to the neonate at birth, via blood transfusion or organ transplantation, or through unsafe needle-sharing practices. Each year, 25 million to 30 million people from nontropical countries visit areas where malaria is endemic, and from 10,000 to 30,000 of them contract malaria. Most malaria infections in the United States occur in persons who have traveled to regions with ongoing malaria transmission. 1-3
Five Plasmodium species cause malaria in humans; of these, Plasmodium falciparum and Plasmodium vivax pose the greatest threat. P falciparum , the deadliest malaria parasite, is most prevalent on the African continent, and P vivax is the dominant malaria parasite in most countries outside of sub-Saharan Africa. The other three species are Plasmodium malariae , Plasmodium ovale , and Plasmodium knowlesi . Typically, about 2,000 malaria cases are diagnosed each year in the U.S., mostly in returned travelers. 4,5 The prevention of mosquito bites is a fundamental goal. Malaria chemoprophylaxis (antimalarial medication taken before, during, and after travel to a country with malaria transmission) depends on individual criteria determined by clinical and laboratory examinations and on travel conditions (destination, season, duration of stay, local living conditions). Standby medication should be prescribed for self-treatment of fever in areas where medical care is not readily available. 6
The CDC publishes U.S. state-level malaria case data annually. Based on the most recent annual malaria-surveillance report (for 2018), the six states with the highest numbers of malaria cases are Maryland (193), Texas (143), California (100), New Jersey (94), Pennsylvania (93), and Florida (70). 7
Types of Malaria
Indigenous Malaria: Indigenous malaria (also known as locally acquired or “homegrown” malaria) is defined by the World Health Organization (WHO) as local mosquito-borne transmission of the disease with no evidence of importation and no direct link to transmission from an imported case. 7 Malaria was successfully eliminated in the U.S. in the early 1950s, with improved sanitation and medical care, technological advances, and widespread insecticide use resulting in successful interruption of malaria transmission. Although endemic transmission of the parasite was halted, competent Anopheles vectors still exist in the U.S.; however, cases of indigenous malaria are rare. 8 Between January 2023 and September 2023, there were nine cases of locally acquired malaria in the U.S.: seven P vivax cases in Sarasota County, Florida; one P vivax case in Cameron County, Texas; and one P falciparum case in Maryland’s Capital Region. All patients are recovering after prompt treatment at area hospitals. No evidence suggests that the cases in these three states are related. 9
Imported Malaria: According to the WHO, imported malaria (also known as travelers’ malaria) refers to an infection that is acquired outside the area where it is diagnosed, with the diagnosis made ≤3 months after the individual returns from an endemic area. However, many countries have adopted their own definitions for imported malaria using different temporal and spatial scales best suited to their surveillance capabilities, local malaria epidemiology, and geographical position in relation to other malaria-endemic countries. 10 Almost all malaria cases in the U.S. are imported and occur in persons traveling from countries with malaria transmission, many from sub-Saharan Africa and South Asia. Those going to sub-Saharan Africa are at greatest risk for contracting malaria and for dying from the infection; however, all travelers to countries where malaria exists may be at risk for infection. 11
Transmission Cycle
In humans, malaria infection is initiated when a female Anopheles mosquito injects Plasmodium sporozoites into the host’s skin during a blood meal. The sporozoites reach the peripheral circulation and migrate to the liver, where they replicate within hepatocytes to form merozoites, which are released into the bloodstream. The merozoites invade RBCs, then develop through the ring, trophozoite, and schizont stages before they form new merozoites, which are released at schizont egress to reinvade new RBCs. 12
Most malaria patients experience headache, often characterized as “pounding” and worsened by standing quickly. Fever, chills, headache, nausea, vomiting, and diarrhea are usually present in some combination. Symptoms usually appear 8 to 25 days after infection, and although they may seem mild at first, they can become more severe if untreated, eventually leading to death. 13,14 Individuals who develop a fever or flulike illness either while traveling in a malaria-risk area or for up to 1 year after returning home should seek immediate medical attention and inform the physician of their travel history. 15
Preventing Imported Malaria
Strategies for malaria prevention in travelers combine three types of interventions: information and education, antimosquito measures, and antimalarial drugs for chemoprophylaxis or standby emergency treatment. 16 The main approach to malaria prevention is denoted by the acronym “ABCD,” wherein A stands for awareness of the risk, B refers to bite prevention, C means the need for chemoprophylaxis, and D stresses the importance of rapid diagnosis and treatment ( TABLE 1 ). 17,18
Risk Assessment and Awareness
Geographical Location and Time of Year: Transmission of malaria is not homogeneously distributed across all countries. In some destinations, malaria transmission occurs throughout the whole country, whereas in others it occurs in defined pockets. If a traveler is going to a highly endemic pocket of a generally low-transmission country during a peak transmission time, this destination may be high-risk for this individual. In some countries with significant seasonal shifts in temperature or rainfall, the transmission intensity may decrease during colder or drier months. Based on knowledge of the climatic conditions in some subtropical or temperate destinations, travelers may choose, for example, mosquito-avoidance measures only during the winter months. Risk-averse travelers should be aware that even in a low-risk situation, just a single bite from an infected female Anopheles mosquito can transmit malaria. 19
Duration of Time in Area and Activities Undertaken: In addition to geographical factors and time of year, risk depends on the time the individual spends in the endemic area and the activities engaged in. For example, camping in a jungle for 3 weeks poses a much higher risk than a 3-day visit to an urban area with air-conditioned accommodations. 18
Bite Avoidance
Mosquito-bite prevention is important for reducing the risk of contracting malaria, and advice on available methods should be offered in pretravel consultations. The peak time for malaria-transmitting mosquitoes to bite is from dusk to dawn; during these times, using repellents and covering up with clothing impregnated with permethrin will help prevent bites. CDC and United Kingdom guidelines recommend the following to all travelers visiting malaria-endemic areas 20,21 :
• Cover all exposed skin with loose, light-colored clothing (long sleeves and long pants) when outside between dusk and dawn. Clothing (including outerwear, hats, and shoes), mosquito nets, and camping gear may be treated with the insecticide permethrin. Clothing impregnated with a concentration of 0.5% permethrin repels and kills ticks, chiggers, mosquitoes, and other biting and nuisance arthropods. Clothing and other items must be treated 24 to 48 hours before being packed for travel to allow time to dry. As with all pesticides, travelers should follow the manufacturer’s instructions on the label. • Apply an insect repellent containing 50% diethyltoluamide (DEET) to exposed skin, following the manufacturer’s instructions (20%-50% DEET provides 6 to 12 hours of protection; no advantage for DEET concentrations >50%). Reapplication may be necessary throughout the evening. Effective alternatives to DEET are picaridin (20% picaridin has a similar duration of protection as 20% DEET), oil of lemon eucalyptus or para-menthane-diol (effective, but frequent reapplication needed), IR3535, and 2-undecanone. 17,18 Used as directed, these repellents registered by the Environmental Protection Agency have been proven safe and effective even for pregnant and breastfeeding women. • On retiring, use a plug-in insecticide vaporizer or knockdown spray to ensure that the room is free of mosquitoes. • When possible, sleep in a well-screened or air-conditioned room. In some situations, use an insecticide-treated mosquito net when outdoors or sleeping in a poorly screened room.
Chemoprophylaxis
In 2018, of 1,788 imported malaria cases reported to the CDC, 1,102 occurred in U.S. residents (civilians and military personnel), and data on chemoprophylaxis use were reported for 974 (88.4%). Approximately 75% (735) of these patients reported not taking any malaria-chemoprophylaxis medication, a larger proportion than was observed in 2017 (829 [71.7%] of 1,157 U.S. residents with prophylaxis-use data). Of the 864 U.S.-resident imported cases with complete information on chemoprophylaxis, 43 (5.0%) adhered to an appropriate regimen, 745 (86.2%) did not take chemoprophylaxis or used a regimen not consistent with CDC treatment guidelines, and 76 (8.8%) took an appropriate preventive medication but skipped doses. The proportion of U.S.-resident cases who adhered to a correct chemoprophylaxis regimen in 2018 was similar to that observed in 2017 (67 patients [6.7%]). 7
To prevent malaria, the CDC recommends that travelers receive chemoprophylaxis. No antimalarial drug is 100% protective, and it must be combined with personal protective measures (insect repellent, long sleeves and pants, sleep setting that is mosquito-free or has an insecticide-treated bed net). 22 The primary goal of malaria chemoprophylaxis is to prevent P falciparum infection, which is primarily responsible for malaria fatalities. 15 The main antimalarial regimens ( TABLE 2 ) are chloroquine, hydroxychloroquine, atovaquone-proguanil, doxycycline, mefloquine, primaquine, and tafenoquine. P falciparum resistance to chloroquine—and, to a lesser degree, other antimalarials—is a worldwide problem; chloroquine’s effectiveness is limited in most locations. 15
Atovaquone-Proguanil: Atovaquone-proguanil is suitable for last-minute travelers because it is started 1 to 2 days before travel; it is also good for shorter trips because it is taken for 7 days, rather than 4 weeks, after leaving the malaria-endemic area. This agent is well tolerated, and side effects are uncommon. It is not for use in pregnant or breastfeeding women or in patients with severe renal impairment. Atovaquone-proguanil is expensive and must be taken daily. The adult tablet contains atovaquone 250 mg + proguanil 100 mg; the pediatric tablet contains atovaquone 62.5 mg + proguanil 25 mg. The adult dosage is 1 tablet orally once daily. Pediatric dosing is weight-based (all dosages, orally once daily): 5 kg to <8 kg, 1/2 pediatric tablet; 8 kg to <10 kg, 3/4 pediatric tablet; 10 kg to <20 kg, 1 pediatric tablet; 20 kg to <30 kg, 2 pediatric tablets; 30 kg to <40 kg, 3 pediatric tablets; ≥40 kg: 1 adult tablet. 23
Chloroquine: Chloroquine is suitable for long trips because it is taken weekly; it is not appropriate for last-minute travelers, as it must be initiated 1 to 2 weeks before travel. It is not for use in patients with known hydroxychloroquine sensitivity. Chloroquine may be used in all trimesters of pregnancy. This drug can exacerbate psoriasis. Chloroquine should not be used in areas with chloroquine and mefloquine resistance. An additional medication may not be needed in patients who are already taking hydroxychloroquine chronically for rheumatologic conditions. The adult dosage is 300 mg base (500 mg salt) orally once weekly; the pediatric dosage is 5 mg/kg base (8.3 mg/kg salt), to a maximum dosage of 300 mg base (500 mg salt), orally once weekly. 23
Hydroxycholoroquine: This agent is an alternative to chloroquine for prophylaxis only in areas with chloroquine-sensitive malaria. Hydroxychloroquine should be initiated 1 to 2 weeks before travel to malaria-endemic areas. It is taken once weekly on the same day each week while the traveler is in malaria-endemic areas and is continued once weekly for 4 weeks after the traveler leaves endemic areas. The adult dosage is 310 mg base (400 mg salt) orally once weekly; the pediatric dosage is 5 mg/kg base (6.5 mg/kg salt), to a maximum dosage of 310 mg base (400 mg salt), orally once weekly. 23
Doxycycline: Doxycycline is a good choice for last-minute travelers because it is started 1 to 2 days before travel. It must be continued another 4 weeks after the traveler leaves malaria-endemic areas. Doxycycline is generally the least expensive antimalarial. Patients taking doxycycline chronically to prevent acne do not need an additional medication. Doxycycline should be used with caution in patients with a known sulfite hypersensitivity. Doxycycline is not for use in pregnant or breastfeeding women, children aged <8 years, or women prone to developing vaginal yeast infections from antibiotic use. Doxycycline has an increased risk of sun sensitivity and can cause upset stomach. The adult dosage is 100 mg orally once daily; for children aged ≥8 years, the dosage is 2.2 mg/kg, to a maximum dosage of 100 mg, orally once daily. 22-24
Mefloquine: Mefloquine is suitable for long trips since it is taken weekly; it is not a good choice for last-minute travelers because it must be initiated > 2 weeks before travel. It may be used in all trimesters of pregnancy and in breastfeeding women. Mefloquine should not be used in areas with mefloquine-resistant Plasmodium species. Mefloquine has known neuropsychiatric side effects. This agent is not for use in patients with certain psychiatric conditions or patients with seizure disorders, and it is not recommended for patients with cardiac conduction abnormalities. The adult dosage is 228 mg base (250 mg salt) orally once weekly. Pediatric dosing is weight-based (all dosages, orally once weekly): < 9 kg, 4.6 mg/kg base (5 mg/kg salt); >9 kg to 19 kg, 1/4 tablet; >19 kg to 30 kg, 1/2 tablet; >30 kg to 45 kg, 3/4 tablet; >45 kg, 1 tablet. 23
Primaquine: Primaquine, one of the most effective medications for preventing P vivax , is a good choice for travelers to areas that have >90% P vivax . It is a good option for shorter trips because it is taken for 7 days—rather than 4 weeks—after the traveler leaves a malaria-endemic area; it is also suitable for last-minute travelers because it is initiated 1 to 2 days before travel. Primaquine should not be used in patients with severe glucose-6-phosphate dehydrogenase (G6PD) deficiency, as it may cause hemolysis and consequently hemolytic anemia. This drug is not for use in pregnant women or breastfeeding women (unless the infant has also been tested for G6PD). It is also contraindicated in patients with severe rheumatoid arthritis or systemic lupus erythematosus because these conditions can lead to granulocytopenia. Primaquine has the potential for upset stomach, rash, and pruritus. The adult dosage is 30 mg base (52.6 mg salt) orally once daily; the pediatric dosage is 0.5 mg/kg base (0.8 mg/kg salt), to a maximum dosage of 30 mg base (52.6 mg salt), orally once daily. In both adults and children, the same dose is used for both primary and terminal prophylaxis, and the therapy duration differs. 22-24
Tafenoquine: Tafenoquine, one of the most effective agents for preventing P vivax malaria, also prevents P falciparum . This medication is suitable for shorter trips because it is taken once, 1 week after leaving the malaria-endemic area, instead of 4 weeks. It is also a good option for last-minute travelers since it is started 3 days before travel. Tafenoquine is contraindicated in patients with G6PD deficiency or those not tested for this condition. Tafenoquine is not indicated for pregnant women, breastfeeding women (unless the infant has also been tested for G6PD deficiency), or children, and it is not recommended for patients who have psychotic disorders. The adult dosage is 200 mg orally weekly. 22,23
Diagnosing malaria is an important part of malaria management; misdiagnosis has potential ripple effects on the treatment, prevention, and ultimate elimination of malaria. Accurate diagnosis enables effective policymaking, planning, and budgeting. 25 The cellular targets of malaria diagnosis in humans include infected RBCs or leukocytes that have ingested parasites. Detectable analytes (chemical constituents) include nucleic acids, antigens, and hemozoin. Additional analytes include human antiparasite antibodies.
Microscopy: This historically has been the standard method for diagnosing malaria, and it remains the primary method in many healthcare settings around the world. 25 Typically, conventional blood-smear microscopy assesses a fraction of 1 mcL of whole blood. Microscopy enables individual species and developmental stages to be identified; however, as the presence of parasites declines or when an infection comprises multiple species, diagnostic accuracy can be difficult to achieve, even for experts. 26
Rapid Diagnostic Tests (RDTs): In locations where microscopy of thin and thick blood films or other methods for detecting Plasmodium parasites in the blood are unavailable, antigen-based RDTs may be an important technique for quick, simple diagnosis. 27 This approach involves applying first a blood specimen to a test card or cassette (depending on the manufacturer), then a buffer reagent. The appearance of specific bands in the test card after 15 to 30 minutes denotes the presence of plasmodium. 25
Molecular Method: These techniques for malaria diagnosis, which include polymerase chain reaction (PCR) and loop-mediated isothermal amplification, have great potential in locations with a low density of infections that can be easily missed by RDTs. PCR amplifies the parasite DNA, resulting in high sensitivity (0.004 parasites/mcL). PCR-based assays exhibit 100-fold greater sensitivity than microscopy, especially for low-parasitemia infections. 25
Education about risk avoidance, prophylactic drugs, repellents, and insecticide-treated bed nets can benefit persons who are traveling to or from endemic areas. 28 Factors that pharmacists should consider when choosing appropriate malaria chemoprophylaxis include tolerability, ease of administration, cost, possible drug-drug interactions, the travel itinerary, and drugs that provide additional protection against other infections. 29 Personal protective measures should be emphasized, as they offer protection against other vector-borne diseases; long, loose, and light-colored clothing should be worn, and repellents should be used appropriately (products containing 50% DEET are recommended). Mosquito nets, ideally impregnated with permethrin, are essential, and individuals traveling to high-risk areas can also treat their clothing with permethrin. 30 Developing an effective malaria vaccine and altering local receptivity (the relative abundance of anopheline vectors and the existence of other climatic and ecological factors favoring transmission) may provide more enduring solutions to the problem of malaria importation. 28
1. Mace KE, Arguin PM, Tan KR. Malaria surveillance—United States, 2015. MMWR Surveill Summ. 2018;67(7):1-28. 2. Croft A. Malaria: prevention in travellers. BMJ . 2000;321(7254):154-160. 3. Maryland Department of Health. Clinician letter: malaria. August 18, 2023. www.mbp.state.md.us/forms/Clinician_Letter_Malaria_08.18.2023.pdf. Accessed November 8, 2023. 4. CDC. CDC malaria program. www.cdc.gov/malaria/resources/cdc_malaria_program_2023.html. Accessed November 8, 2023. 5. World Health Organization. Malaria. www.who.int/news-room/fact-sheets/detail/malaria. Accessed November 8, 2023. 6. Baudon D, Martet G. Paludisme et voyageurs: protection et information [Malaria and travelers: protection and information]. Med Trop (Mars). 1997;57(4):497-500 [French]. 7. Mace KE, Lucchi NW, Tan KR. Malaria surveillance—United States, 2018. MMWR Surveill Summ. 2022;71(8):1-35. 8. Dye-Braumuller KC, Kanyangarara M. Malaria in the USA: how vulnerable are we to future outbreaks? Curr Trop Med Rep. 2021;8(1):43-51. 9. CDC. Locally acquired cases of malaria in Florida, Texas, and Maryland. www.cdc.gov/malaria/new_info/2023/malaria_florida.html. Accessed November 8, 2023. 10. Arisco NJ, Peterka C, Castro MC. Imported malaria definition and minimum data for surveillance. Sci Rep. 2022;12(1):17982. 11. CDC. Malaria information for specific groups. www.cdc.gov/parasites/malaria/specific_groups/index.html. Accessed November 8, 2023. 12. Meibalan E, Marti M. Biology of malaria transmission. Cold Spring Harb Perspect Med. 2017;7(3):a025452. 13. Flegel KM. Symptoms and signs of malaria. Can Med Assoc J. 1976;115(5):409-410. 14. Elflein J. Malaria—statistics & facts. Statista. www.statista.com/topics/3065/malaria/#topicOverview. Accessed November 8, 2023. 15. Schlagenhauf P, Petersen E. Malaria chemoprophylaxis: strategies for risk groups. Clin Microbiol Rev . 2008;21(3):466-472. 16. Loutan L. Malaria: still a threat to travellers. Int J Antimicrob Agents. 2003;21(2):158-163. 17. Behrens RH, Neave PE, Jones CO. Imported malaria among people who travel to visit friends and relatives: is current UK policy effective or does it need a strategic change? Malar J. 2015;14:149. 18. Lalloo DG, Hill DR. Preventing malaria in travellers. BMJ. 2008;336(7657):1362-1366. 19. CDC. Malaria risk assessment for travelers. www.cdc.gov/malaria/travelers/risk_assessment.html. Accessed November 8, 2023. 20. Goodyer L, Song J. Mosquito bite-avoidance attitudes and behaviors in travelers at risk of malaria. J Travel Med. 2014;21(1):33-38. 21. Mutebi JP, Gimnig J. Mosquitoes, ticks & other arthropods. CDC Yellow Book 2024. CDC. wwwnc.cdc.gov/travel/yellowbook/2024/environmental-hazards-risks/mosquitoes-ticks-and-other-arthropods. Accessed November 8, 2023. 22. CDC. Choosing a drug to prevent malaria. www.cdc.gov/malaria/travelers/drugs.html. Accessed November 8, 2023. 23. Tan K, Abanyie F. Malaria: travel-associated infections & diseases: treatment. Yellow Book 2024. CDC. wwwnc.cdc.gov/travel/yellowbook/2024/infections-diseases/malaria#treatment. Accessed November 8, 2023. 24. Tan K, Abanyie F. Malaria: travel-associated infections & diseases. Yellow Book 2024. CDC. wwwnc.cdc.gov/travel/yellowbook/2024/infections-diseases/malaria. Accessed November 8, 2023. 25. Oyegoke OO, Maharaj L, Akoniyon OP, et al. Malaria diagnostic methods with the elimination goal in view. Parasitol Res. 2022;121(7):1867-1885. 26. Zimmerman PA, Howes RE. Malaria diagnosis for malaria elimination. Curr Opin Infect Dis. 2015;28(5):446-454. 27. Gitta B, Kilian N. Diagnosis of malaria parasites Plasmodium spp . in endemic areas: current strategies for an ancient disease. Bioessays. 2020;42(1):e1900138. 28. Sturrock HJW, Roberts KW, Wegbreit J, et al. Tackling imported malaria: an elimination endgame. Am J Trop Med Hyg. 2015;93(1):139-144. 29. Jacquerioz FA, Croft AM. Drugs for preventing malaria in travellers. Cochrane Database Syst Rev. 2009;(4):CD006491. 30. Batchelor T, Gherardin T. Prevention of malaria in travellers. Aust Fam Physician. 2007;36(5):316-320.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact [email protected].

Respiratory Syncytial Virus Infection in Infants and Young Children
Related content, risk of osteomyelitis with joint replacement, coxsackievirus, patients don’t grasp risks of unnecessary antibiotics, management of an emerging multidrug-resistant fungus: candida auris.

- Advertising Contacts
- Editorial Staff
- Professional Organizations
- Submitting a Manuscript
- Privacy Policy
- Classifieds
- U.S. Department of Health & Human Services

- Virtual Tour
- Staff Directory
- En Español
You are here
Nih research matters.
May 7, 2024
Antibody reduces risk of malaria in children
At a glance.
- A single dose of a monoclonal antibody given to children 6 to 10 years of age in Mali proved up to 77% effective at preventing malaria disease.
- Researchers plan to test the antibody in other populations at high risk of death from malaria, including infants and pregnant people.

More than 600,000 people worldwide die from malaria every year, and most are children in Africa. A parasite called Plasmodium , which is spread by mosquitos, causes the disease. Most cases and deaths are caused by the species P. falciparum.
Malaria-prevention strategies include spraying insecticides and administering drugs to help prevent disease. But these are hampered by emerging resistance to insecticides and anti-malarial drugs. The use of preventive drugs also requires frequent contacts with health care providers, which can limit their use in low-resource countries. The recent approval of two new malaria vaccines is a major milestone, but their efficacy is limited and short-lived.
NIH researchers have been working to develop laboratory-made antibodies called monoclonal antibodies to help prevent malaria infections. One, called L9LS, was based on an antibody isolated from a volunteer who received an experimental malaria vaccine. The researchers engineered this candidate antibody to make it last longer in the body.
L9LS binds to a specific protein on the surface of P. falciparum sporozoites, the parasite stage that is transmitted from mosquitos to humans. L9LS binding prevents sporozoites from infecting cells in the liver. This is a necessary step for parasites before infecting blood cells and spreading to other mosquitoes through blood feeding.
In 2022, a research team at NIH’s Vaccine Research Center found that L9LS protected 15 of 17 (88%) adults who had never had malaria from a controlled P. falciparum infection. In a new study, researchers from NIH and the University of Bamako in Mali tested the antibody in children over a six-month malaria season. The results were published on April 26, 2024, in the New England Journal of Medicine .
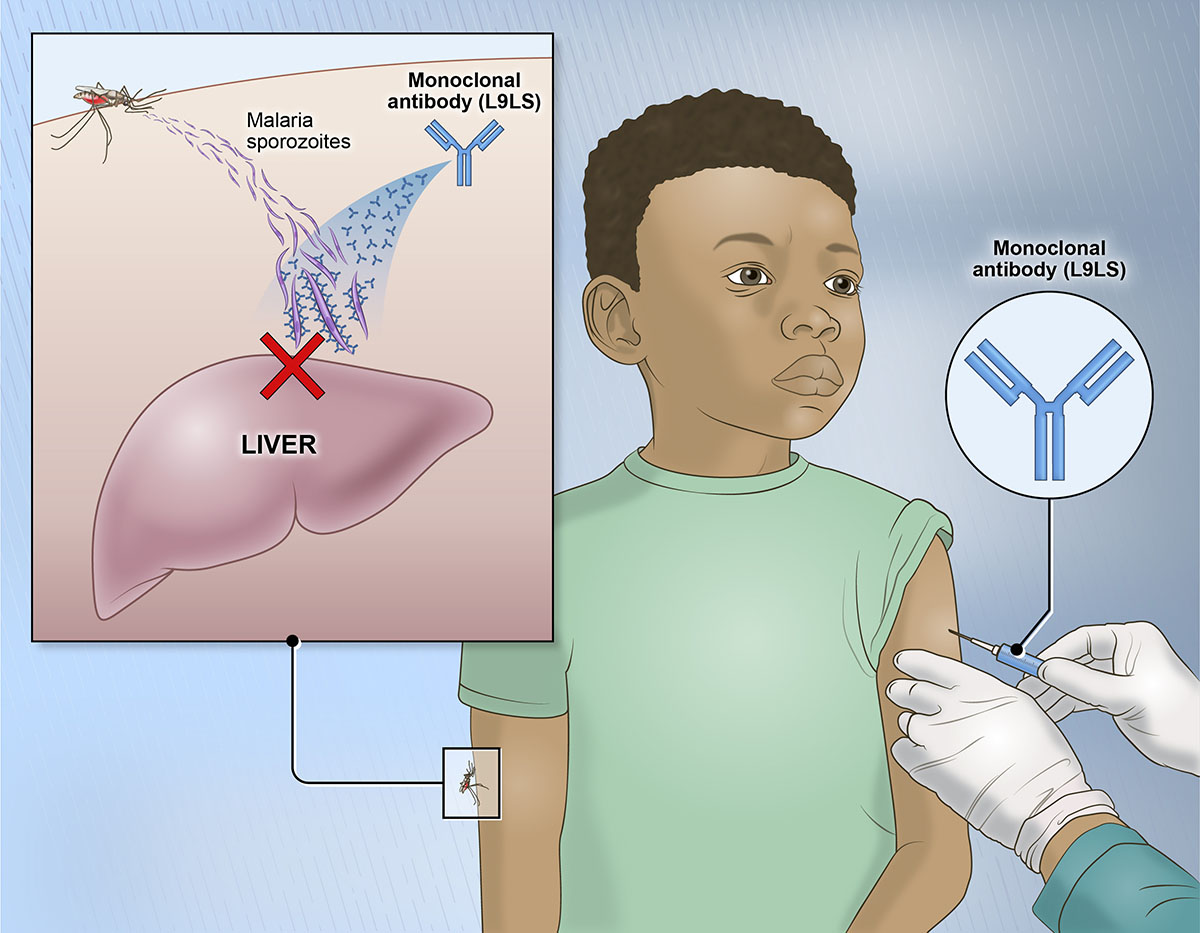
The study took place in Mali, where malaria transmission is extremely high from July to December. The team first tested three different doses of the antibody in adults. This was followed by two different doses in children. When no serious side effects were seen, an additional 225 children joined the study to test whether the antibody could prevent malaria infection and disease.
The children received a single injection with one of two doses of L9LS or a placebo at the beginning of the malaria season. Over the next six months, only 19% of the children who received the higher dose of the antibody and 28% who received the lower dose developed clinical symptoms of malaria. In contrast, almost 60% of those who received the placebo developed symptoms of malaria. The high dose, then, was 77% effective at preventing malaria disease.
L9LS also prevented malaria infections: 40% of those who received the higher dose and 48% of those who received the lower dose had evidence of the parasite in their blood. In contrast, 81% of those who received the placebo had evidence of the parasite. Side effects from injection of the antibody were mild and resolved on their own in all groups.
“A long-acting monoclonal antibody delivered at a single health care visit that rapidly provides high-level protection against malaria in these vulnerable populations would fulfill an unmet public health need,” says Dr. Jeanne Marrazzo, director of NIH’s National Institute of Allergy and Infectious Diseases.
An ongoing clinical trial in Kenya is now testing the antibody in children 5 months to 5 years of age. Researchers are also preparing to test whether the antibody can safely prevent malaria during pregnancy.
Related Links
- Skin Compounds Associated with Attractiveness to Mosquitoes
- Antibody Treatment Protects Adults Against Malaria
- How Mosquitoes Distinguish People from Animals
- Monoclonal Antibody Prevents Malaria in Early Trial
- Malaria Vaccines Provide Strong and Lasting Immunity
- Universal Mosquito Vaccine Tested
- Engineering Malaria Resistance in Mosquitoes
- Drug Prevents Malaria in High-Risk Region
- Battling Bites: Blocking Mosquito-Borne Diseases
References: Subcutaneous Administration of a Monoclonal Antibody to Prevent Malaria. Kayentao K, Ongoiba A, Preston AC, Healy SA, Hu Z, Skinner J, Doumbo S, Wang J, Cisse H, Doumtabe D, Traore A, Traore H, Djiguiba A, Li S, Peterson ME, Telscher S, Idris AH, Adams WC, McDermott AB, Narpala S, Lin BC, Serebryannyy L, Hickman SP, McDougal AJ, Vazquez S, Reiber M, Stein JA, Gall JG, Carlton K, Schwabl P, Traore S, Keita M, Zéguimé A, Ouattara A, Doucoure M, Dolo A, Murphy SC, Neafsey DE, Portugal S, Djimdé A, Traore B, Seder RA, Crompton PD; Mali Malaria mAb Trial Team. N Engl J Med . 2024 May 2;390(17):1549-1559. doi: 10.1056/NEJMoa2312775. Epub 2024 Apr 26. PMID: 38669354.
Funding: NIH’s National Institute of Allergy and Infectious Diseases (NIAID).
Connect with Us
- More Social Media from NIH
Your browser is set to private mode. To continue reading, please log in or subscribe.
Get unlimited access today for only
$9.99 / month
Subscribe now
Malaria still poses health risks
Know the signs, take proactive steps if traveling.
Malaria is caused by a single-celled parasite of the genus Plasmodium. The parasite is transmitted to humans most commonly through mosquito bites. The malaria parasites enter the bloodstream and travel to the liver. When the parasites mature, they leave the liver and infect red blood cells. This is when people typically develop malaria symptoms.
Because the parasites that cause malaria affect red blood cells, people also can be infected by exposure to infected blood, including from mother to unborn child, through blood transfusions and by sharing needles used to inject drugs.
Each year, nearly 290 million people are infected with malaria, and more than 400,000 people die of the disease. The greatest risk factor for developing malaria is to live in or visit areas where the disease is common. These areas include tropical and subtropical regions of sub-Saharan Africa, South and Southeast Asia, the Pacific Islands, Central America, and northern South America. The degree of risk depends on local malaria control, seasonal changes in malaria rates and the precautions you take to prevent mosquito bites.
Symptoms of malaria
Signs and symptoms of malaria typically begin within a few weeks after being bitten by an infected mosquito. However, some types of malaria parasites can lie dormant in your body for up to a year. Some people who have malaria experience cycles of malaria “attacks.” An attack usually starts with shivering and chills, followed by a high fever, then sweating, and finally a return to normal temperature.
Signs and symptoms of malaria can include:
- General feeling of discomfort
- Nausea and vomiting
- Abdominal pain
- Muscle or joint pain
- Rapid breathing
- Rapid heart rate
Treatment, prevention
Malaria is treated with prescription drugs to kill the parasite. The types of drugs and the length of treatment will depend on the type of malaria parasite you have, the severity of your symptoms, your age and whether you’re pregnant.
To reduce malaria infections, world health programs distribute preventive drugs and insecticide-treated bed nets to protect people from mosquito bites. Protective clothing, bed nets and insecticides can protect you while traveling. You also can take preventive medicine before, during and after a trip to a high-risk area.
In 2021, the World Health Organization recommended widespread use of a new malaria vaccine for children. WHO Director-General Dr. Tedros Adhanom Ghebreyesus called the long-awaited vaccine a “breakthrough for science, child health and malaria control,” and said that when combined with existing tools to prevent malaria, tens of thousands of children could be saved each year.
Related Stories
- Perspective
- Open access
- Published: 13 May 2024
Optimal balance of benefit versus risk for tafenoquine in the treatment of Plasmodium vivax malaria
- Raman Sharma 1 ,
- Hema Sharma 2 ,
- Siôn Jones 2 ,
- Isabelle Borghini-Fuhrer 3 ,
- Gonzalo J. Domingo 4 ,
- Rachel A. Gibson 1 ,
- Katie Rolfe 1 ,
- Lionel Tan 2 ,
- Ioana Gabriela Fiţa 2 ,
- Chao Chen 2 ,
- Panayota Bird 2 ,
- Anup Pingle 5 &
- Stephan Duparc 3
Malaria Journal volume 23 , Article number: 145 ( 2024 ) Cite this article
207 Accesses
3 Altmetric
Metrics details
A single 300 mg dose of tafenoquine (an 8-aminoquinoline), in combination with a standard 3-day course of chloroquine, is approved in several countries for the radical cure (prevention of relapse) of Plasmodium vivax malaria in patients aged ≥ 16 years. Despite this, questions have arisen on the optimal dose of tafenoquine. Before the availability of tafenoquine, a 3-day course of chloroquine in combination with the 8-aminoquinoline primaquine was the only effective radical cure for vivax malaria. The World Health Organization (WHO)-recommended standard regimen is 14 days of primaquine 0.25 mg/kg/day or 7 days of primaquine 0.5 mg/kg/day in most regions, or 14 days of primaquine 0.5 mg/kg/day in East Asia and Oceania, however the long treatment courses of 7 or 14 days may result in poor adherence and, therefore, low treatment efficacy. A single dose of tafenoquine 300 mg in combination with a 3-day course of chloroquine is an important advancement for the radical cure of vivax malaria in patients without glucose-6-phosphate dehydrogenase (G6PD) deficiency, as the use of a single-dose treatment will improve adherence. Selection of a single 300 mg dose of tafenoquine for the radical cure of P. vivax malaria was based on collective efficacy and safety data from 33 studies involving more than 4000 trial participants who received tafenoquine, including over 800 subjects who received the 300 mg single dose. The safety profile of single-dose tafenoquine 300 mg is similar to that of standard-dosage primaquine 0.25 mg/kg/day for 14 days. Both primaquine and tafenoquine can cause acute haemolytic anaemia in individuals with G6PD deficiency; severe haemolysis can lead to anaemia, kidney damage, and, in some cases, death. Therefore, relapse prevention using an 8-aminoquinoline must be balanced with the need to avoid clinical haemolysis associated with G6PD deficiency. To minimize this risk, the WHO recommends G6PD testing for all individuals before the administration of curative doses of 8-aminoquinolines. In this article, the authors review key efficacy and safety data from the pivotal trials of tafenoquine and argue that the currently approved dose represents a favourable benefit–risk profile.
Since 2018, single-dose tafenoquine 300 mg has been approved in combination with a standard 3-day course of chloroquine, for the radical cure of Plasmodium vivax malaria in patients aged ≥ 16 years [ 1 ]. Despite approval in several countries, authors of recent articles have questioned the optimal dose of tafenoquine [ 2 , 3 , 4 ]. Indeed one such retrospective meta-analysis conducted by Watson et al., suggested that the recommended adult dose of tafenoquine is insufficient for radical cure in all adults and predicted that the risk of relapse would be substantially reduced with an increased adult dose of 450 mg; in response to this, GSK has published a rebuttal, noting that ‘ a tafenoquine dose increase from 300 mg to 450 mg when co-administered with chloroquine is not supported by available fact-based evidence for the radical cure of P. vivax malaria in adults aged ≥ 16 years ’ [ 4 , 5 ]. Here, the authors summarize data underlying the favourable benefit–risk profile of single-dose tafenoquine 300 mg.
Plasmodium vivax is the most geographically widespread cause of malaria, and is the predominant malarial parasite in South and South-East Asia, Latin America, and North-East Africa, having a substantial global health and economic impact [ 6 , 7 ]. Following the bite of an infected mosquito, P. vivax travels haematogenously to the liver, from where it can exit and cause acute malaria (blood stage). Alternatively, the parasite can remain dormant in the liver as a hypnozoite (liver/hypnozoite stage), reactivating weeks, months, or years later to cause relapses of malaria. Previously considered benign, vivax malaria is responsible for greater morbidity than once understood; it can impact the growth and development of children, as well as cause severe anaemia, pulmonary complications, cerebral malaria, or even death [ 7 , 8 , 9 , 10 , 11 , 12 ].
For the effective management of P. vivax malaria, eradication of both the blood and liver stages is required (radical cure) [ 7 ]. Decades before the approval of tafenoquine, primaquine (an 8-aminoquinoline) in combination with a blood stage treatment (chloroquine or artemisinin-based combination therapy [ACT]), was the only effective regimen targeting hypnozoites and enabling relapse prevention. However, due to its short half-life (6 h), primaquine requires repeated dosing over multiple days for full efficacy, presenting the challenge of ensuring adherence [ 13 , 14 , 15 ]. The World Health Organization (WHO)-recommended standard regimen is 14 days primaquine 0.25 mg/kg/day (equivalent to 15 mg/day) in most regions, or 0.5 mg/kg/day in East Asia and Oceania [ 16 ]. The WHO recently recommended primaquine 0.5 mg/kg/day for 7 days for uncomplicated P. vivax malaria to improve adherence [ 16 ].
Tafenoquine: key findings of clinical efficacy and safety
Tafenoquine is a slowly eliminated 8-aminoquinoline used, in combination with chloroquine, for the radical cure of P. vivax malaria [ 1 ]. Tafenoquine solves the issue of poor adherence to daily primaquine by providing radical cure in a single dose [ 17 ]. The safety profile of single-dose tafenoquine 300 mg is similar to that of standard dose primaquine 0.25 mg/kg/day for 14 days [ 18 ]. Primaquine and tafenoquine can cause acute haemolytic anaemia in individuals with glucose-6-phosphate dehydrogenase (G6PD) deficiency; severe haemolysis can lead to anaemia, kidney damage, and even death [ 19 , 20 ]. G6PD deficiency is a hereditary disorder, with the highest prevalence of G6PD-deficient individuals in malaria-endemic regions [ 18 , 21 ]. As an X-linked disorder, males are either G6PD-deficient or have normal G6PD activity; females can have deficient, intermediate, or normal G6PD activity; G6PD heterozygous females typically have intermediate G6PD activity [ 7 ]. Therefore, relapse prevention through adequate 8-aminoquinoline anti-hypnozoite activity must be balanced with the need to avoid clinical haemolysis due to widespread erythrocyte loss associated with G6PD deficiency [ 20 , 22 ]. To minimize risk, the WHO recommends G6PD testing for all individuals before the administration of curative doses of 8-aminoquinolines, where feasible [ 8 , 23 ].
The haemolytic potential of tafenoquine was assessed in a phase 1 study of 15 G6PD-deficient heterozygous (G6PD Mahidol variant) females, and the haemolytic risk was dose-dependent (ie, a greater maximum haemoglobin decrease was noted as the tafenoquine dose increased from 100 to 300 mg, and dose-limiting toxicity was evident in 3/3 participants at 300 mg). Mean maximum decreases in haemoglobin were –1.72 g/dL, –1.83 g/dL, and –2.83 g/dL for tafenoquine 100, 200, and 300 mg, respectively [ 24 ]. Haemolysis was greatest in participants with the lowest G6PD activity. In addition, the degree of haemolysis associated with single-dose tafenoquine 300 mg was similar to that with primaquine 0.25 mg/kg/day for 14 days [ 24 ]. However, concerns have been raised regarding the risk of haemolysis in G6PD heterozygous females treated with higher doses of primaquine [ 25 , 26 ].
To minimize haemolysis risk, G6PD testing should be performed in all patients who may otherwise be eligible for tafenoquine, and tafenoquine should be withheld from any patient with a phenotypic G6PD test result showing enzyme levels < 70% of normal [ 1 , 27 ]. The 70% threshold for G6PD activity was selected to address the risk of haemolysis in G6PD heterozygous females with intermediate G6PD activity.
Although G6PD heterozygous females may have apparently normal G6PD activity (> 40%) on qualitative G6PD tests, they can still experience haemolysis with higher primaquine doses [ 25 ]. While the long half-life of tafenoquine does not appear to result in a higher risk of haemolysis relative to that of primaquine 0.25 mg/kg/day for 14 days, if haemolysis following tafenoquine or primaquine should progress to more severe acute haemolytic anaemia, the primaquine course can be interrupted, whereas single-dose tafenoquine cannot be stopped once administered [ 24 ]. However, studies have shown that for the G6PD A and G6PD Viangchan variants, continued primaquine administration leads to stabilization of haemoglobin levels—termed ‘resistance phase’—which is a state of low-grade haemolysis resulting from higher G6PD activity in the younger red cell population following acute haemolysis [ 28 , 29 ]. Due to the similarity in haematological parameters, primaquine and tafenoquine may both induce a resistance phase [ 24 ].
Approval of single-dose tafenoquine 300 mg for the radical cure of vivax malaria by regulatory agencies around the world, was based on efficacy and safety data from a comprehensive global clinical development programme of 33 studies involving > 4000 trial participants who received tafenoquine at various doses, including over 800 who received the 300 mg single dose [ 1 , 6 ]. Pivotal data were obtained from three randomized, double-blind studies, in which almost 500 patients with P. vivax malaria received tafenoquine 300 mg: DETECTIVE (ClinicalTrials.gov: NCT01376167) part 1 (phase 2b) [ 30 ] and part 2 (phase 3) [ 31 ]; and GATHER (NCT02216123; phase 3) [ 18 ]. In DETECTIVE part 1 (a dose-ranging study), tafenoquine exposure was a significant predictor of efficacy, and there was only a marginal efficacy gain with a 600 versus 300 mg dose: relapse-free efficacy at 6 months was 91.9% (95% CI 80.0–97.0) and 89.2% (95% CI 77.0–95.0), respectively [ 30 ]. While the study was designed to have sufficient power to detect a 30% treatment difference between each tafenoquine arm and chloroquine alone, it was not designed to have sufficient power to test for a difference between different tafenoquine doses. Population pharmacokinetics modelling demonstrated that single-dose tafenoquine 300 mg provided systemic exposure greater than the clinically relevant breakpoint obtained in a classification and regression tree analysis (area under the concentration–time curve [AUC] of 56.4 μg ⋅ h/mL) in approximately 93% of individuals. Consequently, these individuals would have a high probability (85%) of being relapse-free at 6 months [ 32 ].
In the phase 3 DETECTIVE part 2 and GATHER studies, the recurrence-free rates at 6 months after single-dose tafenoquine 300 mg in the intention-to-treat populations were 62.4% (95% CI 54.9–69.0) and 72.7% (95% CI 64.8–79.2), respectively; the corresponding recurrence-free rates at 4 months were 73.0% (95% CI 66.0–78.9) and 82.3% (95% CI 74.9–87.7), respectively [ 18 , 31 ]. Furthermore, a phase 2b, paediatric study (TEACH; NCT02563496) used a pharmacokinetics bridging design to evaluate tafenoquine dose [ 33 ]. Participants (aged 2–15 years) received tafenoquine according to body weight to achieve the same median AUC as the 300 mg dose in those aged 16 years or older (children weighing > 10–20 kg received 100 or 150 mg; > 20–35 kg received 200 mg; and > 35 kg received 300 mg). The recurrence-free rate at 4 months was 94.7% (95% CI 84.6–98.3) [ 33 ], and the TEACH study supported the approval of tafenoquine for children aged 2–16 years by the Australian Therapeutic Goods Administration in March 2022 and the National Regulatory Agency for Brazil in August 2023 [ 34 , 35 ].
The efficacy and safety of tafenoquine with anti-malarials other than chloroquine have not been established. Dihydroartemisinin–piperaquine is an artemisinin-based combination used as an alternative to chloroquine for the treatment of P. vivax malaria in regions of chloroquine resistance. However, in the INSPECTOR trial (NCT02802501; phase 3) of 150 Indonesian soldiers with normal G6PD activity returning to Java with P. vivax malaria after deployment in Papua, single-dose tafenoquine 300 mg plus a 3-day course of dihydroartemisinin–piperaquine produced relapse-free efficacy of only 21% (95% CI 11–34) at 6 months. Corresponding efficacy rates were 11% (95% CI 4–22) for dihydroartemisinin–piperaquine alone and 52% (95% CI 37–65) for dihydroartemisinin–piperaquine plus 15 mg/day primaquine for 14 days [ 36 ]. Thus, although there was a statistically significant benefit for tafenoquine plus dihydroartemisinin–piperaquine compared with dihydroartemisinin–piperaquine alone in the radical cure of P. vivax malaria, the magnitude of the benefit was not clinically meaningful [ 36 ]. A single post-marketing case from a US safety surveillance study also described lack of efficacy for tafenoquine in combination with another artemisinin-based combination, artemether–lumefantrine [ 37 ]. The reasons for the lack of efficacy for tafenoquine and ACT are still being investigated. No clinically significant pharmacokinetic drug interactions have been identified between tafenoquine and dihydroartemisinin–piperaquine [ 38 ]; however, the possibility of pharmacodynamic interactions with tafenoquine when used with an artemisinin-based combination, instead of chloroquine, needs further exploration. Studies using preclinical models, both in vitro and in vivo, are ongoing, with the aim of exploring possible pharmacodynamic interactions between ACT and 8-aminoquinolines to identify a suitable malaria blood stage treatment (other than chloroquine) that can be used with tafenoquine (Gamo FJ, GSK, personal communication).
A recent modelling study suggests that a higher dose of tafenoquine could result in higher efficacy with little predicted increase in the risk of severe adverse events [ 4 ]. Both the incremental benefits but also the risks of a higher dose are difficult to model and predict, as exemplified recently in reported primaquine studies. Doubling the dose of primaquine to 1 mg/kg/day for 7 days versus 0.5 mg/kg/day for 14 days, thus maintaining the same overall dose, resulted in equivalent efficacy [ 39 , 40 ]. In contrast, both drug-related and drug-unrelated adverse events were significantly higher in the 1 mg/kg/day arm when compared to the 0.5 mg/kg/day dosing [ 39 ] and when compared to Plasmodium falciparum standard of care treatment with a single low dose (0.25 mg/kg) primaquine administered [ 41 ]. A higher risk of haemolysis was also found in females with intermediate G6PD activity [ 26 , 42 ].
Real-world efficacy or effectiveness of a drug is defined by multiple factors beyond its efficacy, but relies heavily on: (i) the compliance of healthcare providers prescribing the drug when and as indicated, and (ii) adherence of the patient to the treatment regimen. Radical cure of P. vivax with primaquine has suffered from shortcomings for both of these reasons [ 43 , 44 ]. Tafenoquine solves attrition in efficacy due to poor adherence. As a result, in real-life conditions when tafenoquine is administered in combination with a point-of-care test for G6PD deficiency, the effectiveness of the 300 mg dose regimen is significantly higher than that of multi-day primaquine, in terms of recurrence-free effectiveness at Day 90 and median time to recurrence [ 45 , 46 ].
Higher doses of tafenoquine are likely to present a higher risk of severe haemolytic events in populations with a significant prevalence of G6PD deficiency, particularly where fragile healthcare systems may result in inappropriate treatment with tafenoquine, in patients with inadequate G6PD activity. The impact of this higher risk as a deterrent on policy for adoption and compliance, therefore reducing overall effectiveness, should be carefully considered.
Radical cure with tafenoquine and primaquine combined with point-of-care testing for G6PD deficiency represents an opportunity in malaria case management in most healthcare settings where P. vivax is prevalent. Learning how to safely scale these effective interventions is essential towards understanding future opportunities to optimize them, including higher doses and weight-based dosing among other opportunities that may arise.
The approved tafenoquine 300 mg dose has undergone clinical trials per regulatory requirement, demonstrating a likely optimal balance between efficacy and safety when dosed with 3 days of chloroquine. As haemolytic risk was shown to be dose dependent, with a dose > 300 mg deviation from the approved dose may impact the risk of haemolysis. An alternative dose should not be recommended without evidence in the field of treatment failure, followed by rigorous clinical trials and safety studies in malaria-endemic regions where tafenoquine may be used. Overall, single-dose tafenoquine 300 mg with a 3-day course of chloroquine is an important advancement for the radical cure of P. vivax malaria in patients without G6PD deficiency, and the use of a single-dose treatment has the potential to improve adherence.
Availability of data and materials
Not applicable.
Abbreviations
Artemisinin-based combination therapy
Area under the concentration–time curve
Glucose-6-phosphate dehydrogenase
World Health Organization
GSK. Krintafel. Highlights of prescribing information. 2018. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/210795s000lbl.pdf .
Hanboonkunupakarn B, White NJ. Advances and roadblocks in the treatment of malaria. Br J Clin Pharmacol. 2022;88:374–82.
Article PubMed Google Scholar
Sharma J, Gautam CS, Singh H, Singh J. Tafenoquine for Plasmodium vivax malaria: concerns regarding efficacy & safety. Indian J Med Res. 2021;154:797–805.
Article CAS PubMed PubMed Central Google Scholar
Watson JA, Commons RJ, Tarning J, Simpson JA, Llanos Cuentas A, Lacerda MVG, et al. The clinical pharmacology of tafenoquine in the radical cure of Plasmodium vivax malaria: an individual patient data meta-analysis. Elife. 2022;11:e83433.
Sharma R, Chen C, Tan L, Rolfe K, Fiţa IG, Jones S, et al. Comment on 'The clinical pharmacology of tafenoquine in the radical cure of Plasmodium vivax malaria: an individual patient data meta-analysis’. Elife. 2024;13:e89263.
Article PubMed PubMed Central Google Scholar
GSK. US FDA approves Krintafel (tafenoquine) for the radical cure of P. vivax malaria. 2018. https://www.gsk.com/en-gb/media/press-releases/us-fda-approves-krintafel-tafenoquine-for-the-radical-cure-of-p-vivax-malaria/ .
Hounkpatin AB, Kreidenweiss A, Held J. Clinical utility of tafenoquine in the prevention of relapse of Plasmodium vivax malaria: a review on the mode of action and emerging trial data. Infect Drug Resist. 2019;12:553–70.
Drysdale M, Tan L, Martin A, Fuhrer IB, Duparc S, Sharma H. Plasmodium vivax in children: hidden burden and conspicuous challenges, a narrative review. Infect Dis Ther. 2022;12:33–51.
Ozen M, Gungor S, Atambay M, Daldal N. Cerebral malaria owing to Plasmodium vivax : case report. Ann Trop Paediatr. 2006;26:141–4.
Fernando SD, Gunawardena DM, Bandara MR, De Silva D, Carter R, Mendis KN, et al. The impact of repeated malaria attacks on the school performance of children. Am J Trop Med Hyg. 2003;69:582–8.
Article CAS PubMed Google Scholar
Lee G, Yori P, Olortegui MP, Pan W, Caulfield L, Gilman RH, et al. Comparative effects of vivax malaria, fever and diarrhoea on child growth. Int J Epidemiol. 2012;41:531–9.
Tan LK, Yacoub S, Scott S, Bhagani S, Jacobs M. Acute lung injury and other serious complications of Plasmodium vivax malaria. Lancet Infect Dis. 2008;8:449–54.
Baird JK. Tafenoquine for travelers’ malaria: evidence, rationale and recommendations. J Travel Med. 2018;25:tay110.
Douglas NM, Poespoprodjo JR, Patriani D, Malloy MJ, Kenangalem E, Sugiarto P, et al. Unsupervised primaquine for the treatment of Plasmodium vivax malaria relapses in southern Papua: a hospital-based cohort study. PLoS Med. 2017;14:e1002379.
Edwards G, McGrath CS, Ward SA, Supanaranond W, Pukrittayakamee S, Davis TM, et al. Interactions among primaquine, malaria infection and other antimalarials in Thai subjects. Br J Clin Pharmacol. 1993;35:193–8.
WHO. Guidelines for malaria - 16 October 2023. 2023. https://app.magicapp.org/#/guideline/LwRMXj/section/j7Wa9j . Accessed May 24, 2023.
White NJ. Tafenoquine - a radical improvement? N Engl J Med. 2019;380:285–6.
Llanos-Cuentas A, Lacerda MVG, Hien TT, Velez ID, Namaik-Larp C, Chu CS, et al. Tafenoquine versus primaquine to prevent relapse of Plasmodium vivax malaria. N Engl J Med. 2019;380:229–41.
Brito-Sousa JD, Santos TC, Avalos S, Fontecha G, Melo GC, Val F, et al. Clinical spectrum of primaquine-induced hemolysis in glucose-6-phosphate dehydrogenase deficiency: a 9-year hospitalization-based study from the Brazilian Amazon. Clin Infect Dis. 2019;69:1440–2.
Baird JK, Battle KE, Howes RE. Primaquine ineligibility in anti-relapse therapy of Plasmodium vivax malaria: the problem of G6PD deficiency and cytochrome P-450 2D6 polymorphisms. Malar J. 2018;17:42.
Howes RE, Piel FB, Patil AP, Nyangiri OA, Gething PW, Dewi M, et al. G6PD deficiency prevalence and estimates of affected populations in malaria endemic countries: a geostatistical model-based map. PLoS Med. 2012;9: e1001339.
Shanks GD. Historical 8-aminoquinoline combinations: not all antimalarial drugs work well together. Am J Trop Med Hyg. 2022;107:964–7.
WHO. Guide to G6PD deficiency rapid diagnostic testing to support P. vivax radical cure. 2018. https://www.who.int/publications/i/item/9789241514286 .
Rueangweerayut R, Bancone G, Harrell EJ, Beelen AP, Kongpatanakul S, Mohrle JJ, et al. Hemolytic potential of tafenoquine in female volunteers heterozygous for glucose-6-phosphate dehydrogenase (G6PD) deficiency (G6PD Mahidol variant) versus G6PD-normal volunteers. Am J Trop Med Hyg. 2017;97:702–11.
Chu CS, Bancone G, Moore KA, Win HH, Thitipanawan N, Po C, et al. Haemolysis in G6PD heterozygous females treated with primaquine for Plasmodium vivax malaria: a nested cohort in a trial of radical curative regimens. PLoS Med. 2017;14:e1002224.
Rajasekhar M, Simpson JA, Ley B, Edler P, Chu CS, Abreha T, et al. Primaquine dose and the risk of haemolysis in patients with uncomplicated Plasmodium vivax malaria: a systematic review and individual patient data meta-analysis. Lancet Infect Dis. 2024;24:184–95.
Chu CS, Freedman DO. Tafenoquine and G6PD: a primer for clinicians. J Travel Med. 2019;26: taz023.
PubMed PubMed Central Google Scholar
Dern RJ, Beutler E, Alving AS. The hemolytic effect of primaquine. II. The natural course of the hemolytic anemia and the mechanism of its self-limited character. J Lab Clin Med. 1954;44:171–6.
CAS PubMed Google Scholar
Kheng S, Muth S, Taylor WR, Tops N, Kosal K, Sothea K, et al. Tolerability and safety of weekly primaquine against relapse of Plasmodium vivax in Cambodians with glucose-6-phosphate dehydrogenase deficiency. BMC Med. 2015;13:203.
Llanos-Cuentas A, Lacerda MV, Rueangweerayut R, Krudsood S, Gupta SK, Kochar SK, et al. Tafenoquine plus chloroquine for the treatment and relapse prevention of Plasmodium vivax malaria (DETECTIVE): a multicentre, double-blind, randomised, phase 2b dose-selection study. Lancet. 2014;383:1049–58.
Lacerda MVG, Llanos-Cuentas A, Krudsood S, Lon C, Saunders DL, Mohammed R, et al. Single-dose tafenoquine to prevent relapse of Plasmodium vivax malaria. N Engl J Med. 2019;380:215–28.
Tenero D, Green JA, Goyal N. Exposure-response analyses for tafenoquine after administration to patients with Plasmodium vivax malaria. Antimicrob Agents Chemother. 2015;59:6188–94.
Velez ID, Hien TT, Green JA, Martin A, Sharma H, Rousell VM, et al. Tafenoquine exposure assessment, safety, and relapse prevention efficacy in children with Plasmodium vivax malaria: open-label, single-arm, non-comparative, multicentre, pharmacokinetic bridging, phase 2 trial. Lancet Child Adolesc Health. 2022;6:86–95.
Medicines for Malaria Venture. Single-dose Kozenis (tafenoquine) approved for children with Plasmodium vivax malaria by Australian Therapeutic Goods Administration. 2022. https://www.mmv.org/newsroom/press-releases/single-dose-kozenis-tafenoquine-approved-children-plasmodium-vivax-malaria .
Medicines for Malaria Venture. Brazil becomes the first malaria-endemic country to register single-dose tafenoquine for children with relapsing malaria. 2023. https://www.mmv.org/newsroom/news-resources-search/brazil-becomes-first-malaria-endemic-country-register-single-dose#:~:text=Brazil%20is%20the%20first%20malaria,vivax%20malaria%20relapses .
Sutanto I, Soebandrio A, Ekawati LL, Chand K, Noviyanti R, Winasti Satyagraha A, et al. Randomised, placebo-controlled, efficacy and safety study of tafenoquine co-administered with dihydroartemisinin-piperaquine for the radical cure of Plasmodium vivax malaria (INSPECTOR). Lancet Infect Dis. 2023;23:1153–63.
GSK. First interim report for a 5-year study to evaluate safety, including hypersensitivity, neuropsychiatric and haematologic adverse reactions, in United States (US) patients taking Krintafel (tafenoquine) for the radical cure of Plasmodium vivax ( P. vivax ) malaria. PIER accession number 7566251. Data on file.
Green JA, Mohamed K, Goyal N, Bouhired S, Hussaini A, Jones SW, et al. Pharmacokinetic interactions between tafenoquine and dihydroartemisinin-piperaquine or artemether-lumefantrine in healthy adult subjects. Antimicrob Agents Chemother. 2016;60:7321–32.
Taylor WRJ, Thriemer K, von Seidlein L, Yuentrakul P, Assawariyathipat T, Assefa A, et al. Short-course primaquine for the radical cure of Plasmodium vivax malaria: a multicentre, randomised, placebo-controlled non-inferiority trial. Lancet. 2019;394:929–38.
Chu CS, Phyo AP, Turner C, Win HH, Poe NP, Yotyingaphiram W, et al. Chloroquine versus dihydroartemisinin-piperaquine with standard high-dose primaquine given either for 7 days or 14 days in Plasmodium vivax malaria. Clin Infect Dis. 2019;68:1311–9.
Thriemer K, Degaga TS, Christian M, Alam MS, Rajasekhar M, Ley B, et al. Primaquine radical cure in patients with Plasmodium falciparum malaria in areas co-endemic for P falciparum and Plasmodium vivax (PRIMA): a multicentre, open-label, superiority randomised controlled trial. Lancet. 2023;402:2101–10.
Chu CS, Bancone G, Nosten F, White NJ, Luzzatto L. Primaquine-induced haemolysis in females heterozygous for G6PD deficiency. Malar J. 2018;17:101.
Recht J, Ashley EA, White NJ. Use of primaquine and glucose-6-phosphate dehydrogenase deficiency testing: divergent policies and practices in malaria endemic countries. PLoS Negl Trop Dis. 2018;12:e0006230.
Mehdipour P, Rajasekhar M, Dini S, Zaloumis S, Abreha T, Adam I, et al. Effect of adherence to primaquine on the risk of Plasmodium vivax recurrence: a WorldWide Antimalarial Resistance Network systematic review and individual patient data meta-analysis. Malar J. 2023;22:306.
Brito M, Rufatto R, Brito-Sousa JD, Murta F, Sampaio V, Balieiro P, et al. Operational effectiveness of tafenoquine and primaquine for the prevention of Plasmodium vivax recurrence in Brazil: a retrospective observational study. Lancet Infect Dis. 2024. https://doi.org/10.1016/S1473-3099(24)00074-4 .
Brito M, Rufatto R, Murta F, Sampaio V, Balieiro P, Baia-Silva D, et al. Operational feasibility of Plasmodium vivax radical cure with tafenoquine or primaquine following point-of-care, quantitative glucose-6-phosphate dehydrogenase testing in the Brazilian Amazon: a real-life retrospective analysis. Lancet Glob Health. 2024;12:e467–77.
Download references
Acknowledgements
Medical writing support was provided by David Murdoch, a contract writer working on behalf of Apollo, and Alex Coulthard, of Apollo, OPEN Health Communications, funded by GSK, in accordance with Good Publication Practice 3 (GPP) guidelines ( www.ismpp.org/gpp-2022 ).
Funding for this article was provided by GSK.
Author information
Authors and affiliations.
Global Health Medicines R&D, GSK Medicines Research Centre, Gunnels Wood Road, Stevenage, Hertfordshire, SG1 2NY, UK
Raman Sharma, Rachel A. Gibson & Katie Rolfe
GSK, Brentford, Middlesex, UK
Hema Sharma, Siôn Jones, Lionel Tan, Ioana Gabriela Fiţa, Chao Chen & Panayota Bird
Medicines for Malaria Venture, Geneva, Switzerland
Isabelle Borghini-Fuhrer & Stephan Duparc
PATH, Seattle, WA, USA
Gonzalo J. Domingo
GSK, Mumbai, India
Anup Pingle
You can also search for this author in PubMed Google Scholar
Contributions
All authors were involved in the development of this manuscript (writing and reviewing). All authors granted their final approval of the manuscript before submission.
Corresponding author
Correspondence to Rachel A. Gibson .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
RS, SJ, RAG, KR, LT, IGF, CC, PB, and AP are employees of, and shareholders in, GSK. HS is a former employee of GSK, a shareholder in GSK, and a current employee of AstraZeneca. GJD reports grant support from the Foreign, Commonwealth & Development office (Grant number: 204139) and Bill & Melinda Gates Foundation (Grant number: OPP1107113). IB-F and SD are employees of Medicines for Malaria Venture.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Sharma, R., Sharma, H., Jones, S. et al. Optimal balance of benefit versus risk for tafenoquine in the treatment of Plasmodium vivax malaria. Malar J 23 , 145 (2024). https://doi.org/10.1186/s12936-024-04924-z
Download citation
Received : 31 August 2023
Accepted : 29 March 2024
Published : 13 May 2024
DOI : https://doi.org/10.1186/s12936-024-04924-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Plasmodium vivax malaria
- Radical cure
- Tafenoquine
- Benefit–risk
Malaria Journal
ISSN: 1475-2875
- Submission enquiries: [email protected]

IMAGES
COMMENTS
Begin 1-2 days before travel, daily during travel, and for 7 days after leaving. Good for last-minute travelers because the drug is started 1-2 days before traveling to an area where malaria transmission occurs. Some people prefer to take a daily medicine. Good choice for shorter trips because you only have to take the medicine for 7 days after ...
For trips of short duration, some people would rather not take medication for 4 weeks after travel. Not a good choice for last-minute travelers because drug needs to be started 1-2 weeks prior to travel. Doxycycline. Begin 1 - 2 days before travel, daily during travel, and for 4 weeks after leaving. Adults: 100 mg daily.
Malaria pills lower your chance of getting sick with the tropical disease. Although they aren't 100% effective, they are an important way to reduce your chances of getting malaria while traveling.
This information is intended for travelers who reside in the United States. Travelers from other countries may find this information helpful; however, because malaria prevention recommendations and the availability of antimalarial drugs vary, travelers from other countries should consult health care providers in their respective countries.
To diagnose malaria, your doctor will likely review your medical history and recent travel, conduct a physical exam, and order blood tests. Blood tests can indicate: The presence of the parasite in the blood, to confirm that you have malaria. Which type of malaria parasite is causing your symptoms. If your infection is caused by a parasite ...
Malaria is a sometimes fatal disease that is transmitted through an infected mosquito bite. Common symptoms usually include chills, fever, and sweating. Individuals who are traveling to certain countries should make sure they have taken precautions with antimalarial drugs and medication.
Dosage Recommendations for Prevention and Treatment of Malaria. Chloroquine base 5 mg/kg body weight base by mouth, up to 300 mg once weekly (equivalent to 7.5 mg/kg body weight chloroquine phosphate). Start 1-2 weeks before leaving, take weekly while away, and then take once weekly for 4 weeks after returning home.
Malaria is a disease caused by a parasite. Mosquitoes spread the parasite to people when they bite them. Malaria symptoms usually appear within in 7 to 30 days but can take up to one year to develop. Symptoms may include high fevers and shaking chills, flu-like illness. Without treatment, malaria can cause severe illness and death.
Travelers who decline malaria prophylaxis or who will be traveling to remote areas with limited access to health care may be prescribed a three-day supply of presumptive malaria treatment before ...
Malaria is a treatable disease. Artemisinin-based combination therapies (ACTs) are the most effective antimalarial medicines available today and the mainstay of recommended treatment for Plasmodium falciparum malaria, the deadliest malaria parasite globally. ACTs combine 2 active pharmaceuticals with different mechanisms of action, including derivates of artemisinin extracted from the plant ...
For women that chose to travel to a malaria-endemic region, emphasis on mosquito avoidance measures and chemoprophylaxis should be provided. In the US, malaria chemoprophylaxis in pregnancy is limited to mefloquine and chloroquine. In some countries, atovaquone/proguanil is used for either treatment or prophylaxis of malaria during pregnancy.
To discuss the specific malaria medication you need and other travel-related health precautions you should take, consult with a travel medicine expert well before your trip. Malaria is a disease caused by a parasite that is transmitted to humans through the bites of infected mosquitoes. People who have malaria usually get a high fever, headache ...
Three Different Sites of Action of Antimalarial Drugs. Antimalarial chemoprophylaxis with atovaquone-proguanil and doxycycline should begin 1 to 2 days before travel to areas where malaria is ...
available in the United States by prescription only. It is sold under the brand name Malarone and it is also sold as a generic medicine in two sizes: Adult tablet: 250mg atovaquone plus 100mg proguanil Pediatric tablet: 62.5mg atovaquone plus 25mg proguanil. Atovaquone-proguanil can be prescribed for either prevention or treatment of malaria.
Because malaria symptoms may not appear for some time, your doctor may advise you to continue taking malaria vaccine medications for up to four weeks post-travel. A travel malaria medication regimen is recommended for those traveling to India and most African nations, including but not limited to: South Africa. Ghana. Kenya.
Doxycycline: Doxycycline is a good choice for last-minute travelers because it is started 1 to 2 days before travel. It must be continued another 4 weeks after the traveler leaves malaria-endemic areas. Doxycycline is generally the least expensive antimalarial.
contracting malaria, inadequate training in travel medicine for the prescriber, 100 mg/day starting 2 days before exposure and continuing for 4 weeks fear of drug side effects, cost issues, and ... by treatment' may be prescribed by the travel medicine specialist. This is however not routinely recommended, requires intensive education, and ...
Malaria symptoms occur at least 7 to 9 days after infection. Fever in the first week of travel is unlikely to be malaria, but . any illness should be promptly evaluated. It is critical to get immediate treatment of malaria. Any traveler . who becomes ill with a fever or flu-like illness while traveling
More than 600,000 people worldwide die from malaria every year, and most are children in Africa. A parasite called Plasmodium, which is spread by mosquitos, causes the disease.Most cases and deaths are caused by the species P. falciparum.. Malaria-prevention strategies include spraying insecticides and administering drugs to help prevent disease.
Both adults and children should take one dose of hydroxychloroquine per week starting at least 1 week before traveling to the area where malaria transmission occurs. They should take one dose per week while there, and for 4 consecutive weeks after leaving. The weekly dosage for adults is 310mg base (400mg salt). Center for Global Health.
Malaria is caused by a single-celled parasite of the genus Plasmodium. The parasite is transmitted to humans most commonly through mosquito bites. The malaria parasites enter the bloodstream and trave
Tafenoquine is a slowly eliminated 8-aminoquinoline used, in combination with chloroquine, for the radical cure of P. vivax malaria [].Tafenoquine solves the issue of poor adherence to daily primaquine by providing radical cure in a single dose [].The safety profile of single-dose tafenoquine 300 mg is similar to that of standard dose primaquine 0.25 mg/kg/day for 14 days [].