
- Board of Directors
- Healthcare Advisory Committee
- Become a Member
- Anal Cancer
- Appendix Cancer
- Bile Duct Cancer
- Esophageal Cancer
- Colorectal Cancer
- Gallbladder Cancer
- Liver Cancer
- Pancreatic Cancer
- Rectal Cancer
- Small Intestine Cancer
- Stomach Cancer
- Our Members
- Resource Library
A Patient’s Journey Battling Stomach Cancer
In honor of Stomach Cancer Awareness Month Martha Raymond, Executive Director of the GI Cancers Alliance, interviews Steve Melen, a Stomach Cancer survivor and patient advocate, about his story with Stomach Cancer when he was diagnosed at 37 years old, having a new baby, a new job and other pressures in his life. Steve even features his new book, Killer Graces: My path from pain to power and breakthrough living, where he talks about his journey of adoption, cancer, addiction, and breakthrough living.
Learn more about Debbie’s Dream Foundation .
Gastric Cancer Treatment (PDQ®)–Health Professional Version
General Information About Gastric Cancer
Incidence and Mortality
Estimated new cases and deaths from gastric cancer in the United States in 2024:[ 1 ]
- New cases: 26,890.
- Deaths: 10,880.
Epidemiology
Management of adenocarcinoma histology, which accounts for 90% to 95% of all gastric malignancies, is discussed in this summary. Changing epidemiological patterns in the United States regarding the anatomical location of esophagogastric cancers show a trend of decreased occurrence of distal or noncardia gastric cancers.[ 2 ] However, in people aged 25 to 39 years, there has been an increase in the incidence of noncardia gastric cancers from 0.27 cases per 100,000 individuals (1977–1981) to 0.45 cases per 100,000 individuals (2002–2006).[ 2 ] Additional studies are needed to confirm the observed increases in noncardia gastric cancers in this specific age group.
In contrast to the overall stable trend for noncardia gastric cancers, earlier studies demonstrated an increased incidence of adenocarcinomas of the gastric cardia of 4% to 10% per year from the mid-1970s to the late 1980s.[ 3 ] Similarly, the incidence of gastroesophageal junction adenocarcinomas increased sharply, from 1.22 cases per 100,000 individuals (1973–1978) to 2.00 cases per 100,000 individuals (1985–1990).[ 4 ] Since that time, the incidence has remained steady at 1.94 cases per 100,000 individuals (2003–2008).[ 4 ] More recent data demonstrate that the incidence of gastric cardia cancers has been relatively stable, although an increase has been observed, from 2.4 cases per 100,000 individuals (1977–1981) to 2.9 cases per 100,000 individuals (2001–2006) in the White population.[ 2 ] The reasons for these temporal changes in incidence are unclear.
Risk Factors
In the United States, gastric cancer ranks 14th in incidence among the major types of cancer. While the precise etiology is unknown, acknowledged risk factors for gastric cancer include the following:[ 5 - 7 ]
- Helicobacter pylori gastric infection.
- Advanced age.
- Diet low in fruits and vegetables.
- Diet high in salted, smoked, or preserved foods.
- Chronic atrophic gastritis.
- Intestinal metaplasia.
- Pernicious anemia.
- Gastric adenomatous polyps.
- Family history of gastric cancer.
- Cigarette smoking.
- Ménétrier disease (giant hypertrophic gastritis).
- Epstein-Barr virus infection.
- Familial syndromes (including familial adenomatous polyposis).
Prognosis and Survival
The prognosis of patients with gastric cancer is related to tumor extent and includes both nodal involvement and direct tumor extension beyond the gastric wall.[ 8 , 9 ] Tumor grade may also provide some prognostic information.[ 10 ]
In localized distal gastric cancer, more than 50% of patients can be cured. However, early-stage disease accounts for only 10% to 20% of all cases diagnosed in the United States. The remaining patients present with metastatic disease in either regional or distant sites. The 5-year overall survival rate in these patients ranges from almost no survival for patients with disseminated disease to almost 50% survival for patients with localized distal gastric cancers confined to resectable regional disease. Even with apparent localized disease, the 5-year survival rate of patients with proximal gastric cancer is only 10% to 15%. Although the treatment of patients with disseminated gastric cancer may result in palliation of symptoms and some prolongation of survival, long remissions are uncommon.
Gastrointestinal stromal tumors occur most commonly in the stomach. For more information, see Gastrointestinal Stromal Tumors Treatment .
- American Cancer Society: Cancer Facts and Figures 2024. American Cancer Society, 2024. Available online . Last accessed January 17, 2024.
- Anderson WF, Camargo MC, Fraumeni JF, et al.: Age-specific trends in incidence of noncardia gastric cancer in US adults. JAMA 303 (17): 1723-8, 2010. [PUBMED Abstract]
- Blot WJ, Devesa SS, Kneller RW, et al.: Rising incidence of adenocarcinoma of the esophagus and gastric cardia. JAMA 265 (10): 1287-9, 1991. [PUBMED Abstract]
- Buas MF, Vaughan TL: Epidemiology and risk factors for gastroesophageal junction tumors: understanding the rising incidence of this disease. Semin Radiat Oncol 23 (1): 3-9, 2013. [PUBMED Abstract]
- Kurtz RC, Sherlock P: The diagnosis of gastric cancer. Semin Oncol 12 (1): 11-8, 1985. [PUBMED Abstract]
- Scheiman JM, Cutler AF: Helicobacter pylori and gastric cancer. Am J Med 106 (2): 222-6, 1999. [PUBMED Abstract]
- Fenoglio-Preiser CM, Noffsinger AE, Belli J, et al.: Pathologic and phenotypic features of gastric cancer. Semin Oncol 23 (3): 292-306, 1996. [PUBMED Abstract]
- Siewert JR, Böttcher K, Stein HJ, et al.: Relevant prognostic factors in gastric cancer: ten-year results of the German Gastric Cancer Study. Ann Surg 228 (4): 449-61, 1998. [PUBMED Abstract]
- Nakamura K, Ueyama T, Yao T, et al.: Pathology and prognosis of gastric carcinoma. Findings in 10,000 patients who underwent primary gastrectomy. Cancer 70 (5): 1030-7, 1992. [PUBMED Abstract]
- Adachi Y, Yasuda K, Inomata M, et al.: Pathology and prognosis of gastric carcinoma: well versus poorly differentiated type. Cancer 89 (7): 1418-24, 2000. [PUBMED Abstract]
Cellular Classification of Gastric Cancer
The two major types of gastric adenocarcinoma are the following:
- Intestinal.
Intestinal adenocarcinomas are well differentiated, and the cells tend to arrange themselves in tubular or glandular structures. The terms tubular, papillary, and mucinous are assigned to the various types of intestinal adenocarcinomas. Rarely, adenosquamous cancers can occur.
Diffuse adenocarcinomas are undifferentiated or poorly differentiated, and they lack a gland formation. Clinically, diffuse adenocarcinomas can give rise to infiltration of the gastric wall (i.e., linitis plastica).
Some tumors can have mixed features of intestinal and diffuse types.
Stage Information for Gastric Cancer
Ajcc prognostic stage groups and tnm definitions.
The American Joint Committee on Cancer (AJCC) has designated staging by TNM (tumor, node, metastasis) classification to define gastric cancer.[ 1 ]
Pathological (pTNM)
- Stomach. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. Springer; 2017, pp. 203–20.
Treatment Option Overview
Radical surgery represents the standard form of therapy that has curative intent. However, the incidences of local failure in the tumor bed and regional lymph nodes, and distant failures via hematogenous or peritoneal routes, remain high.[ 1 ] As such, comprehensive staging and evaluation with a multidisciplinary team to determine roles of neoadjuvant, perioperative, and adjuvant combination chemotherapy, surgery, and external-beam radiation therapies should be considered.
Investigators in Europe evaluated the role of perioperative chemotherapy without radiation therapy.[ 2 ] Initially, in a randomized phase III trial ( MRC-ST02 [NCT00002615]), patients with stage II or higher adenocarcinoma of the stomach or of the lower third of the esophagus were assigned to receive three cycles of epirubicin, cisplatin, and continuous infusion fluorouracil (5-FU) (ECF) before and after surgery or to receive surgery alone. Compared with the surgery group, the perioperative chemotherapy group had a significantly higher overall survival (OS) (hazard ratio [HR] death , 0.75; 95% confidence interval [CI], 0.60–0.93; P = .009).[ 2 ][ Level of evidence A1 ]
In addition, in the randomized phase III AIO-FLOT4 trial (NCT01216644), patients with resectable disease that was stage T2 or higher and/or node positive received either perioperative epirubicin, cisplatin, and 5-FU or capecitabine (ECF/ECX) (three cycles before and after surgery) or perioperative docetaxel, oxaliplatin, and 5-FU/leucovorin (FLOT) (four 2-week cycles before and after surgery). OS was significantly increased from 35 months with ECF/ECX to 50 months with FLOT (HR, 0.77; 95% CI, 0.63–0.94; P = .012).[ 3 ]
In a phase III Intergroup trial ( SWOG-9008 [NCT01197118]), 559 patients with completely resected stage IB to stage IV (M0) adenocarcinoma of the stomach and gastroesophageal junction were randomly assigned to receive either surgery alone or surgery plus postoperative chemotherapy (5-FU and leucovorin) and concurrent radiation therapy (45 Gy). With a median follow-up of more than 10 years, a significant survival benefit was reported for patients who received adjuvant combined modality therapy.[ 4 ][ Level of evidence A1 ] Median OS was 35 months for the adjuvant chemoradiation therapy group and 27 months for the surgery-alone arm ( P = .0046). Median relapse-free survival was 27 months in the chemoradiation arm compared with 19 months in the surgery-alone arm ( P < .001).
Gastroesophageal junction cancers may be treated like esophageal cancers and are best managed under the care of a multidisciplinary team. For more information, see Esophageal Cancer Treatment .
Capecitabine and Fluorouracil Dosing
The DPYD gene encodes an enzyme that catabolizes pyrimidines and fluoropyrimidines, like capecitabine and fluorouracil. An estimated 1% to 2% of the population has germline pathogenic variants in DPYD , which lead to reduced DPD protein function and an accumulation of pyrimidines and fluoropyrimidines in the body.[ 5 , 6 ] Patients with the DPYD*2A variant who receive fluoropyrimidines may experience severe, life-threatening toxicities that are sometimes fatal. Many other DPYD variants have been identified, with a range of clinical effects.[ 5 - 7 ] Fluoropyrimidine avoidance or a dose reduction of 50% may be recommended based on the patient's DPYD genotype and number of functioning DPYD alleles.[ 8 - 10 ] DPYD genetic testing costs less than $200, but insurance coverage varies due to a lack of national guidelines.[ 11 ] In addition, testing may delay therapy by 2 weeks, which would not be advisable in urgent situations. This controversial issue requires further evaluation.[ 12 ]
- Gunderson LL, Sosin H: Adenocarcinoma of the stomach: areas of failure in a re-operation series (second or symptomatic look) clinicopathologic correlation and implications for adjuvant therapy. Int J Radiat Oncol Biol Phys 8 (1): 1-11, 1982. [PUBMED Abstract]
- Cunningham D, Allum WH, Stenning SP, et al.: Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 355 (1): 11-20, 2006. [PUBMED Abstract]
- Al-Batran SE, Homann N, Pauligk C, et al.: Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet 393 (10184): 1948-1957, 2019. [PUBMED Abstract]
- Smalley SR, Benedetti JK, Haller DG, et al.: Updated analysis of SWOG-directed intergroup study 0116: a phase III trial of adjuvant radiochemotherapy versus observation after curative gastric cancer resection. J Clin Oncol 30 (19): 2327-33, 2012. [PUBMED Abstract]
- Sharma BB, Rai K, Blunt H, et al.: Pathogenic DPYD Variants and Treatment-Related Mortality in Patients Receiving Fluoropyrimidine Chemotherapy: A Systematic Review and Meta-Analysis. Oncologist 26 (12): 1008-1016, 2021. [PUBMED Abstract]
- Lam SW, Guchelaar HJ, Boven E: The role of pharmacogenetics in capecitabine efficacy and toxicity. Cancer Treat Rev 50: 9-22, 2016. [PUBMED Abstract]
- Shakeel F, Fang F, Kwon JW, et al.: Patients carrying DPYD variant alleles have increased risk of severe toxicity and related treatment modifications during fluoropyrimidine chemotherapy. Pharmacogenomics 22 (3): 145-155, 2021. [PUBMED Abstract]
- Amstutz U, Henricks LM, Offer SM, et al.: Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for Dihydropyrimidine Dehydrogenase Genotype and Fluoropyrimidine Dosing: 2017 Update. Clin Pharmacol Ther 103 (2): 210-216, 2018. [PUBMED Abstract]
- Henricks LM, Lunenburg CATC, de Man FM, et al.: DPYD genotype-guided dose individualisation of fluoropyrimidine therapy in patients with cancer: a prospective safety analysis. Lancet Oncol 19 (11): 1459-1467, 2018. [PUBMED Abstract]
- Lau-Min KS, Varughese LA, Nelson MN, et al.: Preemptive pharmacogenetic testing to guide chemotherapy dosing in patients with gastrointestinal malignancies: a qualitative study of barriers to implementation. BMC Cancer 22 (1): 47, 2022. [PUBMED Abstract]
- Brooks GA, Tapp S, Daly AT, et al.: Cost-effectiveness of DPYD Genotyping Prior to Fluoropyrimidine-based Adjuvant Chemotherapy for Colon Cancer. Clin Colorectal Cancer 21 (3): e189-e195, 2022. [PUBMED Abstract]
- Baker SD, Bates SE, Brooks GA, et al.: DPYD Testing: Time to Put Patient Safety First. J Clin Oncol 41 (15): 2701-2705, 2023. [PUBMED Abstract]
Treatment of Stage 0 Gastric Cancer
Treatment options for stage 0 gastric cancer.
Treatment options for stage 0 gastric cancer include the following:
- Endoscopic mucosal resection (EMR) .
Stage 0 is gastric cancer confined to mucosa. Experience in Japan, where stage 0 is diagnosed frequently, indicates that more than 90% of patients treated by gastrectomy with lymphadenectomy will survive beyond 5 years. An American series confirmed these results.[ 1 ]
Endoscopic mucosal resection (EMR)
EMR has been studied in Japan and throughout Asia in patients with early-stage tumors with good-risk features (Tis or T1a, diameter ≤2 cm, predominantly differentiated type, without ulcerative findings) that have a lower risk of nodal metastasis. Intramucosal tumors have a lower risk of nodal metastasis than submucosal tumors.[ 2 ] Careful patient selection by the above criteria, treatment with an experienced endoscopist, and close surveillance should be considered.
Evidence (EMR):
- Of the 405 patients with intramucosal disease, 278 underwent complete resection, with 2% local recurrence treated with curative intent and 100% disease-free survival at a median follow-up of 38 months.
- In those with resections that were incomplete or not evaluable, 18 of 127 patients had a local recurrence and underwent curative surgery.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
- Green PH, O'Toole KM, Slonim D, et al.: Increasing incidence and excellent survival of patients with early gastric cancer: experience in a United States medical center. Am J Med 85 (5): 658-61, 1988. [PUBMED Abstract]
- Japanese Gastric Cancer Association: Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer 20 (1): 1-19, 2017. [PUBMED Abstract]
- Ono H, Kondo H, Gotoda T, et al.: Endoscopic mucosal resection for treatment of early gastric cancer. Gut 48 (2): 225-9, 2001. [PUBMED Abstract]
Treatment of Stage I Gastric Cancer
Treatment options for stage i gastric cancer.
Treatment options for stage I gastric cancer include the following:
- Distal subtotal gastrectomy (if the lesion is not in the fundus or at the cardioesophageal junction).
- Proximal subtotal gastrectomy or total gastrectomy, both with distal esophagectomy (if the lesion involves the cardia). These tumors often involve the submucosal lymphatics of the esophagus.
- Total gastrectomy (if the tumor involves the stomach diffusely or arises in the body of the stomach and extends to within 6 cm of the cardia or distal antrum).
Regional lymphadenectomy is recommended with all of the above procedures. Splenectomy is not routinely performed.[ 1 ]
- Endoscopic mucosal resection (EMR) for select patients with stage IA gastric cancer.
- Postoperative chemoradiation therapy or perioperative chemotherapy for patients with node-positive (T1 N1) and muscle-invasive (T2 N0) disease.[ 2 , 3 ]
- Neoadjuvant chemoradiation (under clinical evaluation).[ 4 ]
Surgical resection
Surgical resection including regional lymphadenectomy is the treatment of choice for patients with stage I gastric cancer.[ 1 ] If the lesion is not in the cardioesophageal junction and does not diffusely involve the stomach, subtotal gastrectomy is the procedure of choice, because it has been demonstrated to provide equivalent survival when compared with total gastrectomy and is associated with decreased morbidity.[ 5 ][ Level of evidence A1 ] When the lesion involves the cardia, proximal subtotal gastrectomy or total gastrectomy (including a sufficient length of esophagus) may be performed with curative intent. If the lesion diffusely involves the stomach, total gastrectomy is required. At a minimum, surgical resection includes greater and lesser curvature perigastric regional lymph nodes. In patients with stage I gastric cancer, perigastric lymph nodes may contain cancer.
EMR has been studied in Japan and throughout Asia in patients with early-stage tumors with good-risk features (Tis or T1a, diameter ≤2 cm, predominantly differentiated type, without ulcerative findings) that have a lower risk of nodal metastasis. Intramucosal tumors have a lower risk of nodal metastasis than submucosal tumors.[ 6 ] Careful patient selection by the above criteria, treatment with an experienced endoscopist, and close surveillance should be considered.
Postoperative chemoradiation therapy
In patients with node-positive (T1 N1) and muscle-invasive (T2 N0) disease, postoperative chemoradiation therapy may be considered.
Evidence (postoperative chemoradiation therapy):
- With more than 10 years of follow-up, median survival was 35 months for the adjuvant chemoradiation therapy group and 27 months for the surgery-alone arm ( P = .0046).
- Median relapse-free survival was 27 months in the chemoradiation arm compared with 19 months in the surgery-alone arm ( P < .001). Improvement was primarily seen for locoregional recurrence risk (improvement from 47% for surgery vs. 29% for chemoradiation).[ 2 ] However, only 36 patients in the trial had stage IB tumors (18 patients in each arm).[ 8 ]
Because the prognosis is relatively favorable for patients with completely resected stage IB disease, the effectiveness of adjuvant chemoradiation therapy for this group is less clear.
Perioperative chemotherapy
Investigators in Europe evaluated the role of perioperative chemotherapy without radiation therapy.[ 9 ]
Evidence (perioperative chemotherapy):
- Median overall survival was 50 months with FLOT and 35 months with ECF/ECX (hazard ratio, 0.77; 95% confidence interval, 0.63–0.94; P = .012).
- Margin-free resection in the FLOT group was 85% versus 78% in the ECF/ECX group ( P = .0162).
- Toxicity rates were similar between groups (26% required hospitalizations in the ECF/ECX group and 25% in the FLOT group). However, types of side effects differed, with increased nausea, thromboembolic events, and anemia in the ECF/ECX group versus higher rates of grade 3/4 infections, neutropenia, diarrhea, and neuropathy in the FLOT group.
- Brennan MF, Karpeh MS: Surgery for gastric cancer: the American view. Semin Oncol 23 (3): 352-9, 1996. [PUBMED Abstract]
- Ajani JA, Winter K, Okawara GS, et al.: Phase II trial of preoperative chemoradiation in patients with localized gastric adenocarcinoma (RTOG 9904): quality of combined modality therapy and pathologic response. J Clin Oncol 24 (24): 3953-8, 2006. [PUBMED Abstract]
- Bozzetti F, Marubini E, Bonfanti G, et al.: Subtotal versus total gastrectomy for gastric cancer: five-year survival rates in a multicenter randomized Italian trial. Italian Gastrointestinal Tumor Study Group. Ann Surg 230 (2): 170-8, 1999. [PUBMED Abstract]
- Kelsen DP: Postoperative adjuvant chemoradiation therapy for patients with resected gastric cancer: intergroup 116. J Clin Oncol 18 (21 Suppl): 32S-4S, 2000. [PUBMED Abstract]
Treatment of Stages II and III Gastric Cancer
Treatment options for stages ii and iii gastric cancer.
Treatment options for stage II gastric cancer and stage III gastric cancer include the following:
- Proximal subtotal gastrectomy or total gastrectomy (if the lesion involves the cardia).
- Total gastrectomy (if the tumor involves the stomach diffusely or arises in the body of the stomach and extends to within 6 cm of the cardia).
- Perioperative chemotherapy .[ 2 ]
- Postoperative (adjuvant) chemoradiation therapy .[ 3 ]
- Postoperative (adjuvant) chemotherapy .
- Neoadjuvant chemoradiation therapy (under clinical evaluation).[ 4 ]
- Perioperative chemotherapy and immunotherapy regimens (under clinical evaluation).
No randomized trials of adjuvant chemoradiation versus perioperative chemotherapy have been undertaken.
All newly diagnosed patients with stages II and III gastric cancer should consider clinical trials.
Because of the high risk of locoregional and distant recurrence, perioperative and postoperative therapy should be considered in addition to surgery.
Surgical resection with regional lymphadenectomy is the treatment of choice for patients with stages II and III gastric cancer; all eligible patients undergo surgery.[ 1 ] If the lesion is not in the cardioesophageal junction and does not diffusely involve the stomach, subtotal gastrectomy is the procedure of choice. When the lesion involves the cardia, proximal subtotal gastrectomy or total gastrectomy may be performed with curative intent. If the lesion diffusely involves the stomach, total gastrectomy and appropriate lymph node resection may be required. The role of extended lymph node (D2) dissection is uncertain [ 5 ] and in some series is associated with increased morbidity.[ 6 , 7 ] As many as 15% of selected stage III patients can be cured by surgery alone, particularly if lymph node involvement is minimal (<7 lymph nodes).
Investigators in Europe evaluated the role of perioperative chemotherapy without radiation therapy.[ 2 ]
- Median overall survival (OS) was 50 months with FLOT and 35 months with ECF/ECX (hazard ratio [HR], 0.77; 95% confidence interval [CI], 0.63–0.94; P = .012).
- Compared with the surgery group, the perioperative chemotherapy group had a significantly higher likelihood of progression-free survival (HR progression , 0.66; 95% CI, 0.53–0.81; P < .001) and of OS (HR death , 0.75; 95% CI, 0.60–0.93; P = .009).
- The 5-year OS rate was 36.3% (95% CI, 29.5%‒43.0%) for the perioperative chemotherapy group and 23% (95% CI, 16.6%‒29.4%) for the surgery group.[ 2 ][ Level of evidence A1 ]
Postoperative (adjuvant) chemoradiation therapy
Postoperative chemoradiation therapy may be considered for patients with stages II and III gastric cancer who have not received neoadjuvant therapy.
Evidence (postoperative [adjuvant] chemoradiation therapy):
- With more than 10 years of follow-up, median survival was 35 months for the adjuvant chemoradiation therapy arm and 27 months for the surgery-alone arm ( P = .0046).
- Median relapse-free survival was 27 months in the chemoradiation arm compared with 19 months in the surgery-alone arm ( P < .001). Improvement was primarily seen for locoregional recurrence risk (improvement from 47% for surgery vs. 29% for chemoradiation).[ 3 ] However, only 36 patients in the trial had stage IB tumors (18 patients in each arm).[ 9 ]
- The 5-year OS rate was 44% in both arms.
- Median OS was 43 months in the chemotherapy arm and 37 months in the chemoradiotherapy group (95% CI, 0.84–1.22; P = .90).
Postoperative (adjuvant) chemotherapy
Investigators in Europe evaluated the role of postoperative chemotherapy without radiation therapy.[ 2 ]
Evidence (postoperative [adjuvant] chemotherapy):
- The 3-year OS rate was 80.1% in the S-1 group and 70.1% in the surgery-only group. The HR death in the S-1 group, as compared with the surgery-only group, was 0.68 (95% CI, 0.52–0.87; P = .003).[ 12 ][ Level of evidence A1 ]
- The 3-year disease-free survival rate was 74% in the chemotherapy group and 59% in the surgery-alone group (HR, 0.56; 95% CI, 0.44–0.72; P < .0001).
- The 3-year OS rate was 83% in the chemotherapy group and 78% in the surgery-alone group (HR, 0.72; 95% CI, 0.52–1.00; P = .0493).[ 13 ][ Level of evidence A1 ]
- Further follow-up is anticipated.
- Kitamura K, Yamaguchi T, Sawai K, et al.: Chronologic changes in the clinicopathologic findings and survival of gastric cancer patients. J Clin Oncol 15 (12): 3471-80, 1997. [PUBMED Abstract]
- Bonenkamp JJ, Songun I, Hermans J, et al.: Randomised comparison of morbidity after D1 and D2 dissection for gastric cancer in 996 Dutch patients. Lancet 345 (8952): 745-8, 1995. [PUBMED Abstract]
- Cuschieri A, Fayers P, Fielding J, et al.: Postoperative morbidity and mortality after D1 and D2 resections for gastric cancer: preliminary results of the MRC randomised controlled surgical trial.The Surgical Cooperative Group. Lancet 347 (9007): 995-9, 1996. [PUBMED Abstract]
- Fuchs CS, Niedzwiecki D, Mamon HJ, et al.: Adjuvant Chemoradiotherapy With Epirubicin, Cisplatin, and Fluorouracil Compared With Adjuvant Chemoradiotherapy With Fluorouracil and Leucovorin After Curative Resection of Gastric Cancer: Results From CALGB 80101 (Alliance). J Clin Oncol 35 (32): 3671-3677, 2017. [PUBMED Abstract]
- Cats A, Jansen EPM, van Grieken NCT, et al.: Chemotherapy versus chemoradiotherapy after surgery and preoperative chemotherapy for resectable gastric cancer (CRITICS): an international, open-label, randomised phase 3 trial. Lancet Oncol 19 (5): 616-628, 2018. [PUBMED Abstract]
- Sakuramoto S, Sasako M, Yamaguchi T, et al.: Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med 357 (18): 1810-20, 2007. [PUBMED Abstract]
- Bang YJ, Kim YW, Yang HK, et al.: Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet 379 (9813): 315-21, 2012. [PUBMED Abstract]
Treatment of Stage IV, Inoperable, and Recurrent Gastric Cancer
Treatment options for stage iv, inoperable, and recurrent gastric cancer.
Treatment options for stage IV, inoperable, and recurrent gastric cancer , including patients with medically or surgically unresectable disease, include a combination of cytotoxic therapies, targeted therapies, immunotherapies, and palliative locoregional therapies.
Patients with metastatic gastric adenocarcinoma should consider undergoing testing for HER2 amplification, defective mismatch repair (dMMR) (immunohistochemistry [IHC] staining), or microsatellite instability (MSI) (polymerase chain reaction), along with programmed death ligand 1 (PD-L1) combined positive score (CPS score in the United States).
- Chemotherapy with immunotherapy: fluorouracil (5-FU) or capecitabine combined with oxaliplatin and nivolumab.
- 5-FU combined with either epirubicin and cisplatin, etoposide and leucovorin, doxorubicin and methotrexate, leucovorin and irinotecan, or docetaxel and cisplatin or oxaliplatin.[ 1 - 7 ]
- A taxane (docetaxel or paclitaxel) and either cisplatin or carboplatin.
- 5-FU and cisplatin.
- Capecitabine and oxaliplatin.[ 8 ]
- 5-FU or capecitabine.[ 9 , 10 ]
- A taxane (either docetaxel or paclitaxel).
- Nivolumab with chemotherapy .
- Trastuzumab with chemotherapy .
- Palliative chemotherapy .
- Ramucirumab with or without chemotherapy .
- Pembrolizumab for patients with dMMR or MSI-high (MSI-H) tumors.
- Trastuzumab deruxtecan for patients with HER2-positive tumors (3+ on IHC or 2+ on IHC with a positive FISH).
- Trifluridine and tipiracil .
- Endoluminal laser therapy, endoluminal stent placement, or gastrojejunostomy may be helpful to patients with gastric obstruction.[ 11 ]
- Palliative radiation therapy may alleviate bleeding, pain, and obstruction.
- Palliative resection is reserved for patients with continued bleeding or obstruction.
- Regorafenib with nivolumab (under clinical evaluation).
- Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (under clinical evaluation).
Treatment with poly (ADP-ribose) polymerase (PARP) inhibitors and hepatocyte growth factor inhibitors have not shown efficacy at this time, but combination studies are under way.
First-line palliative systemic therapy for patients with HER2-negative tumors
Palliative chemotherapy with or without immunotherapy.
Standard chemotherapy versus best supportive care for patients with metastatic gastric cancer has been tested in several clinical trials, and there is general agreement that patients who receive chemotherapy live for several months longer on average than patients who receive supportive care.[ 12 - 14 ][ Level of evidence A1 ] During the last 20 years, multiple randomized studies evaluating different treatment regimens (monotherapy vs. combination [doublet and triplet] chemotherapy) have been performed in patients with metastatic gastric cancer with no clear consensus as to the best management approach. A meta-analysis of these studies demonstrated a hazard ratio (HR) of 0.83 for overall survival (OS) (95% confidence interval [CI], 0.74–0.93) in favor of combination chemotherapy.[ 15 ] The addition of immune checkpoint inhibitors to oxaliplatin-based chemotherapy has shown further OS benefit.
Evidence (palliative chemotherapy):
- The group who received ECF had a significantly longer median survival (8.9 vs. 5.7 months, P = .0009) than the FAMTX group.[ 16 ][ Level of evidence A1 ]
- In a second trial that compared ECF with mitomycin, cisplatin, and 5-FU (MCF), there was no statistically significant difference in median survival (9.4 vs. 8.7 months, P = .315).[ 2 ][ Level of evidence A1 ]
- The trial demonstrated noninferior median OS in patients treated with capecitabine rather than 5-FU (HR death , 0.86; 95% CI, 0.82–0.99) and in patients treated with oxaliplatin in place of cisplatin (HR death , 0.92; 95% CI, 0.80–1.10)
- Patients who received DCF experienced a significantly longer TTP (5.6 months; 95% CI, 4.9–5.9; vs. 3.7 months; 95% CI, 3.4–4.5; HR, 1.47; 95% CI, 1.19–1.82; log-rank P < .001; risk reduction, 32%).
- The median OS was significantly longer for patients who received DCF compared with patients who received CF (9.2 months; 95% CI, 8.4–10.6; vs. 8.6 months; 95% CI, 7.2–9.5; HR, 1.29; 95% CI, 1.0–1.6; log-rank P = .02; risk reduction, 23%).[ 17 ][ Level of evidence A1 ]
- The toxicity rates were high in both arms.[ 18 ]
- Febrile neutropenia was more common in patients who received DCF (29% vs. 12%), and the death rate on the study was 10.4% for patients on the DCF arm and 9.4% for patients on the CF arm.
- Grades 3 and 4 neutropenia occurred in 35% to 43% of patients on all arms, but severe nausea and vomiting was more common in patients in the CF arm and occurred in 26% of those patients.[ 3 ][ Level of evidence B3 ]
Phase II studies that evaluated irinotecan-based or oxaliplatin-based regimens demonstrated similar response rates and TTP to those reported in trials using ECF or CF, but the former may be less toxic.[ 19 - 24 ] There are conflicting data regarding relative efficacy of any one regimen.
First-line palliative systemic therapy for patients with HER2-positive tumors (3+ on IHC or 2+ on IHC with a positive FISH)
Immunotherapy with chemotherapy, nivolumab with chemotherapy.
Nivolumab may be considered in combination with chemotherapy for patients with advanced or metastatic gastric cancer regardless of PD-L1 CPS status.[ 25 ]
Evidence (nivolumab with chemotherapy):
- For all patients (regardless of PD-L1 status), the median OS was 14.0 months (95% CI, 12.6–15.0) in the nivolumab-plus-chemotherapy arm compared with 11.3 months (95 % CI, 10.6–12.3) in the chemotherapy-alone arm (HR, 0.77; 99.3% CI, 0.64–0.92; P < .0001).
- Patients with tumors with PD-L1 CPS greater than 5 had a median OS of 14.4 months (95% CI, 13.1–16.2) in the nivolumab-plus-chemotherapy arm compared with 11.1 months (95% CI, 10.0−12.1) in the chemotherapy-alone arm (HR, 0.71; 98.4% CI, 0.59–0.86; P = .0001).
- Grades 3 and 4 adverse events occurred in 462 patients in the combination arm and in 341 patients in the chemotherapy-alone arm.[ 25 ][ Level of evidence A1 ]
Trastuzumab with chemotherapy
Trastuzumab may be combined with pembrolizumab and chemotherapy (5-FU and cisplatin or oxaliplatin with capecitabine) as treatment for patients with HER2-positive metastatic gastric adenocarcinoma. For patients who do not tolerate pembrolizumab, trastuzumab may be combined with cisplatin and 5-FU or capecitabine. HER2 testing is recommended for patients with metastatic disease.[ 26 ]
Evidence (trastuzumab and pembrolizumab with chemotherapy):
- The objective response rate at the first interim analysis was 74.4% (95% CI, 66.2%–81.6%) for patients in the pembrolizumab arm and 51.9% (95% CI, 43.0%–60.7%) for patients in the placebo arm.[ 26 ][ Level of evidence B3 ]
- Adverse events grade 3 or higher were observed in 57.1% of patients in the pembrolizumab arm (including 33.6% of patients with immune-related reactions) and 57.4% of patients in the placebo arm.
Evidence (trastuzumab):
- The median OS was 13.8 months (95% CI, 12–16) in patients assigned to trastuzumab and 11.1 months (95% CI, 10–13) in patients assigned to chemotherapy alone (HR, 0.74; 95% CI, 0.60–0.91; P = .0046).[ 27 ][ Level of evidence A1 ]
- There was no significant difference in rates of any adverse event, and cardiotoxic effects were equally rare in both arms.
Pembrolizumab with chemotherapy
The combination of pembrolizumab and chemotherapy has not shown superiority over chemotherapy alone.
Evidence (pembrolizumab with chemotherapy):
- The final results did not show superiority of pembrolizumab or pembrolizumab with chemotherapy over chemotherapy alone.
- However, when selected for a PD-L1 CPS of ten or greater, median OS was 17.4 months (95% CI, 9.1−23.1) in the pembrolizumab-alone arm compared with 10.8 months (95% CI, 8.5−13.8) in the chemotherapy-alone arm (HR, 0.69; 95% CI, 0.49−0.97). The prespecified statistical analysis plan did not test this difference further.
Second-line palliative systemic therapy
There is no standard treatment option for patients who develop disease progression after first-line palliative chemotherapy. Accepted regimens include paclitaxel with or without ramucirumab, docetaxel, and irinotecan with or without 5-FU/leucovorin. Pembrolizumab is approved for the treatment of patients with dMMR or MSI-H tumors, and trastuzumab deruxtecan is approved for patients with HER2-positive gastric cancer.
Palliative chemotherapy
- The median OS was 5.3 months in the group that received salvage chemotherapy and 3.8 months in the group that received best supportive care (HR, 0.657; P = .007).
- There was no difference in median OS between docetaxel and irinotecan (5.2 months vs. 6.5 months, P = .116).[ 29 ][ Level of evidence A1 ]
Ramucirumab with or without chemotherapy
Ramucirumab is a fully humanized monoclonal antibody directed against the vascular endothelial growth factor receptor-2.
Evidence (ramucirumab):
- Patients who were assigned to ramucirumab had a significantly improved median OS of 5.2 months compared with a median OS of 3.8 months in patients who were assigned to the placebo (HR, 0.776; P = .047).
- Rates of hypertension were higher in the ramucirumab group than in the placebo group.[ 30 ][ Level of evidence A1 ]
Ramucirumab is an acceptable treatment in patients with cisplatin- or 5-FU‒refractory, stage IV, gastric cancer.
- Patients who were assigned to ramucirumab had a significant improvement in median OS of 9.6 months compared with a median OS of 7.4 months in patients who were assigned to a placebo (HR, 0.807; P = .017).
- Grade 3 or higher neutropenia, fatigue, hypertension, and abdominal pain were more common in the ramucirumab group.[ 31 ][ Level of evidence A1 ]
The combination of paclitaxel and ramucirumab is an acceptable second-line chemotherapy regimen in patients with stage IV gastric or gastroesophageal junction cancer.
Pembrolizumab for patients with dMMR or MSI-H tumors
Evidence (pembrolizumab for patients with dMMR or MSI-H tumors):
- In a phase II study of pembrolizumab 200 mg IV every 3 weeks in patients with colon cancer with or without dMMR, and noncolorectal cancer with dMMR, the immune-related objective response rate was 71% (5 of 7 patients). On the basis of these data, pembrolizumab was approved for patients with dMMR solid tumors that have progressed after previous treatment and who have no satisfactory alternative treatment options.[ 32 ]
Trastuzumab deruxtecan for patients with HER2-positive tumors (3+ on IHC or 2+ on IHC with a positive FISH)
Trastuzumab deruxtecan is an antibody-drug conjugate combining an anti-HER2 antibody with a topoisomerase I inhibitor via a cleavable tetrapeptide-based linker. The U.S. Food and Drug Administration (FDA) approved trastuzumab deruxtecan for patients with locally advanced or metastatic gastric or gastroesophageal junction cancer that is HER2-positive who have previously received a trastuzumab-based regimen.
Evidence (trastuzumab deruxtecan for patients with HER2-positive tumors):
- The objective response rate was 51% for patients who received trastuzumab deruxtecan and 14% for patients who received chemotherapy ( P < .001).
- The OS was 12.5 months for patients who received trastuzumab deruxtecan and 8.4 months for patients who received chemotherapy (HR, 0.59; 95% CI, 0.39–0.88, P = .02).[ 33 ][ Level of evidence A1 ]
- Common side effects of trastuzumab deruxtecan included neutropenia (grade 3–4 in 51% of patients, with six patients developing neutropenic fever) and anemia (grade 3–4 in 38% of patients). Twelve patients (10%) developed drug-related interstitial lung disease.
Third-line palliative systemic therapy
Trifluridine and tipiracil.
Trifluridine and tipiracil is an oral cytotoxic therapy approved by the FDA for third-line treatment of patients with metastatic gastric or gastroesophageal junction cancer.
Evidence (trifluridine and tipiracil):
- The median OS was 5.7 months (95% CI, 4.8−6.2) in patients who received trifluridine and tipiracil, compared with 3.6 months (95% CI, 3.1−4.1) in patients who received placebo (HR, 0.69; 95% CI, 0.57−0.85; P = .00058).
- The objective response rate was 4% (disease control rate, 44%) in the trifluridine and tipiracil arm, compared with 2% (disease control rate, 14%) in the placebo arm.
- Grade 3 or higher adverse events occurred in 80% of patients treated with trifluridine and tipiracil, compared with 58% of patients who received placebo.
Immunotherapy
Pembrolizumab.
While pembrolizumab was previously evaluated as third-line treatment for patients with gastric and gastroesophageal junction cancers and a PD-L1 CPS of one or greater, this approval was withdrawn after updates to first-line therapy using combination chemotherapy and programmed death 1 (PD-1) inhibitors.
Nivolumab has been approved by the Japanese Ministry of Health, Labor, and Welfare for treatment of advanced gastric cancer, regardless of PD-L1 CPS status.
Evidence (nivolumab):
- The median OS was 5.26 months (95% CI, 4.60–6.37) in the nivolumab group compared with 4.14 months (95% CI, 3.42–4.86) in the placebo group.
- Serious treatment-related adverse events occurred in 10% of the patients.[ 35 ][ Level of evidence A1 ]
- Waters JS, Norman A, Cunningham D, et al.: Long-term survival after epirubicin, cisplatin and fluorouracil for gastric cancer: results of a randomized trial. Br J Cancer 80 (1-2): 269-72, 1999. [PUBMED Abstract]
- Ross P, Nicolson M, Cunningham D, et al.: Prospective randomized trial comparing mitomycin, cisplatin, and protracted venous-infusion fluorouracil (PVI 5-FU) With epirubicin, cisplatin, and PVI 5-FU in advanced esophagogastric cancer. J Clin Oncol 20 (8): 1996-2004, 2002. [PUBMED Abstract]
- Vanhoefer U, Rougier P, Wilke H, et al.: Final results of a randomized phase III trial of sequential high-dose methotrexate, fluorouracil, and doxorubicin versus etoposide, leucovorin, and fluorouracil versus infusional fluorouracil and cisplatin in advanced gastric cancer: A trial of the European Organization for Research and Treatment of Cancer Gastrointestinal Tract Cancer Cooperative Group. J Clin Oncol 18 (14): 2648-57, 2000. [PUBMED Abstract]
- Van Cutsem E, Moiseyenko VM, Tjulandin S, et al.: Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol 24 (31): 4991-7, 2006. [PUBMED Abstract]
- Ajani JA, Ota DM, Jackson DE: Current strategies in the management of locoregional and metastatic gastric carcinoma. Cancer 67 (1 Suppl): 260-5, 1991. [PUBMED Abstract]
- Guimbaud R, Louvet C, Ries P, et al.: Prospective, randomized, multicenter, phase III study of fluorouracil, leucovorin, and irinotecan versus epirubicin, cisplatin, and capecitabine in advanced gastric adenocarcinoma: a French intergroup (Fédération Francophone de Cancérologie Digestive, Fédération Nationale des Centres de Lutte Contre le Cancer, and Groupe Coopérateur Multidisciplinaire en Oncologie) study. J Clin Oncol 32 (31): 3520-6, 2014. [PUBMED Abstract]
- Cunningham D, Starling N, Rao S, et al.: Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 358 (1): 36-46, 2008. [PUBMED Abstract]
- Cullinan SA, Moertel CG, Fleming TR, et al.: A comparison of three chemotherapeutic regimens in the treatment of advanced pancreatic and gastric carcinoma. Fluorouracil vs fluorouracil and doxorubicin vs fluorouracil, doxorubicin, and mitomycin. JAMA 253 (14): 2061-7, 1985. [PUBMED Abstract]
- Ohtsu A, Shimada Y, Shirao K, et al.: Randomized phase III trial of fluorouracil alone versus fluorouracil plus cisplatin versus uracil and tegafur plus mitomycin in patients with unresectable, advanced gastric cancer: The Japan Clinical Oncology Group Study (JCOG9205). J Clin Oncol 21 (1): 54-9, 2003. [PUBMED Abstract]
- Ell C, Hochberger J, May A, et al.: Coated and uncoated self-expanding metal stents for malignant stenosis in the upper GI tract: preliminary clinical experiences with Wallstents. Am J Gastroenterol 89 (9): 1496-500, 1994. [PUBMED Abstract]
- Murad AM, Santiago FF, Petroianu A, et al.: Modified therapy with 5-fluorouracil, doxorubicin, and methotrexate in advanced gastric cancer. Cancer 72 (1): 37-41, 1993. [PUBMED Abstract]
- Pyrhönen S, Kuitunen T, Nyandoto P, et al.: Randomised comparison of fluorouracil, epidoxorubicin and methotrexate (FEMTX) plus supportive care with supportive care alone in patients with non-resectable gastric cancer. Br J Cancer 71 (3): 587-91, 1995. [PUBMED Abstract]
- Glimelius B, Ekström K, Hoffman K, et al.: Randomized comparison between chemotherapy plus best supportive care with best supportive care in advanced gastric cancer. Ann Oncol 8 (2): 163-8, 1997. [PUBMED Abstract]
- Wagner AD, Grothe W, Haerting J, et al.: Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol 24 (18): 2903-9, 2006. [PUBMED Abstract]
- Webb A, Cunningham D, Scarffe JH, et al.: Randomized trial comparing epirubicin, cisplatin, and fluorouracil versus fluorouracil, doxorubicin, and methotrexate in advanced esophagogastric cancer. J Clin Oncol 15 (1): 261-7, 1997. [PUBMED Abstract]
- Ajani JA, Moiseyenko VM, Tjulandin S, et al.: Clinical benefit with docetaxel plus fluorouracil and cisplatin compared with cisplatin and fluorouracil in a phase III trial of advanced gastric or gastroesophageal cancer adenocarcinoma: the V-325 Study Group. J Clin Oncol 25 (22): 3205-9, 2007. [PUBMED Abstract]
- Ilson DH: Docetaxel, cisplatin, and fluorouracil in gastric cancer: does the punishment fit the crime? J Clin Oncol 25 (22): 3188-90, 2007. [PUBMED Abstract]
- Ilson DH, Saltz L, Enzinger P, et al.: Phase II trial of weekly irinotecan plus cisplatin in advanced esophageal cancer. J Clin Oncol 17 (10): 3270-5, 1999. [PUBMED Abstract]
- Beretta E, Di Bartolomeo M, Buzzoni R, et al.: Irinotecan, fluorouracil and folinic acid (FOLFIRI) as effective treatment combination for patients with advanced gastric cancer in poor clinical condition. Tumori 92 (5): 379-83, 2006 Sep-Oct. [PUBMED Abstract]
- Pozzo C, Barone C, Szanto J, et al.: Irinotecan in combination with 5-fluorouracil and folinic acid or with cisplatin in patients with advanced gastric or esophageal-gastric junction adenocarcinoma: results of a randomized phase II study. Ann Oncol 15 (12): 1773-81, 2004. [PUBMED Abstract]
- Bouché O, Raoul JL, Bonnetain F, et al.: Randomized multicenter phase II trial of a biweekly regimen of fluorouracil and leucovorin (LV5FU2), LV5FU2 plus cisplatin, or LV5FU2 plus irinotecan in patients with previously untreated metastatic gastric cancer: a Federation Francophone de Cancerologie Digestive Group Study--FFCD 9803. J Clin Oncol 22 (21): 4319-28, 2004. [PUBMED Abstract]
- Ajani JA, Baker J, Pisters PW, et al.: CPT-11 plus cisplatin in patients with advanced, untreated gastric or gastroesophageal junction carcinoma: results of a phase II study. Cancer 94 (3): 641-6, 2002. [PUBMED Abstract]
- Cavanna L, Artioli F, Codignola C, et al.: Oxaliplatin in combination with 5-fluorouracil (5-FU) and leucovorin (LV) in patients with metastatic gastric cancer (MGC). Am J Clin Oncol 29 (4): 371-5, 2006. [PUBMED Abstract]
- Janjigian YY, Shitara K, Moehler M, et al.: First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet 398 (10294): 27-40, 2021. [PUBMED Abstract]
- Janjigian YY, Kawazoe A, Yañez P, et al.: The KEYNOTE-811 trial of dual PD-1 and HER2 blockade in HER2-positive gastric cancer. Nature 600 (7890): 727-730, 2021. [PUBMED Abstract]
- Bang YJ, Van Cutsem E, Feyereislova A, et al.: Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 376 (9742): 687-97, 2010. [PUBMED Abstract]
- Shitara K, Van Cutsem E, Bang YJ, et al.: Efficacy and Safety of Pembrolizumab or Pembrolizumab Plus Chemotherapy vs Chemotherapy Alone for Patients With First-line, Advanced Gastric Cancer: The KEYNOTE-062 Phase 3 Randomized Clinical Trial. JAMA Oncol 6 (10): 1571-1580, 2020. [PUBMED Abstract]
- Kang JH, Lee SI, Lim do H, et al.: Salvage chemotherapy for pretreated gastric cancer: a randomized phase III trial comparing chemotherapy plus best supportive care with best supportive care alone. J Clin Oncol 30 (13): 1513-8, 2012. [PUBMED Abstract]
- Fuchs CS, Tomasek J, Yong CJ, et al.: Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 383 (9911): 31-9, 2014. [PUBMED Abstract]
- Wilke H, Muro K, Van Cutsem E, et al.: Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol 15 (11): 1224-35, 2014. [PUBMED Abstract]
- Le DT, Uram JN, Wang H, et al.: PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med 372 (26): 2509-20, 2015. [PUBMED Abstract]
- Shitara K, Bang YJ, Iwasa S, et al.: Trastuzumab Deruxtecan in Previously Treated HER2-Positive Gastric Cancer. N Engl J Med 382 (25): 2419-2430, 2020. [PUBMED Abstract]
- Shitara K, Doi T, Dvorkin M, et al.: Trifluridine/tipiracil versus placebo in patients with heavily pretreated metastatic gastric cancer (TAGS): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 19 (11): 1437-1448, 2018. [PUBMED Abstract]
- Kang YK, Boku N, Satoh T, et al.: Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 390 (10111): 2461-2471, 2017. [PUBMED Abstract]
Latest Updates to This Summary (01/26/2024)
The PDQ cancer information summaries are reviewed regularly and updated as new information becomes available. This section describes the latest changes made to this summary as of the date above.
Updated statistics with estimated new cases and deaths for 2024 (cited American Cancer Society as reference 1).
Added Capecitabine and Fluorouracil Dosing as a new subsection.
This summary is written and maintained by the PDQ Adult Treatment Editorial Board , which is editorially independent of NCI. The summary reflects an independent review of the literature and does not represent a policy statement of NCI or NIH. More information about summary policies and the role of the PDQ Editorial Boards in maintaining the PDQ summaries can be found on the About This PDQ Summary and PDQ® Cancer Information for Health Professionals pages.
About This PDQ Summary
Purpose of this summary.
This PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about the treatment of gastric cancer. It is intended as a resource to inform and assist clinicians in the care of their patients. It does not provide formal guidelines or recommendations for making health care decisions.
Reviewers and Updates
This summary is reviewed regularly and updated as necessary by the PDQ Adult Treatment Editorial Board , which is editorially independent of the National Cancer Institute (NCI). The summary reflects an independent review of the literature and does not represent a policy statement of NCI or the National Institutes of Health (NIH).
Board members review recently published articles each month to determine whether an article should:
- be discussed at a meeting,
- be cited with text, or
- replace or update an existing article that is already cited.
Changes to the summaries are made through a consensus process in which Board members evaluate the strength of the evidence in the published articles and determine how the article should be included in the summary.
The lead reviewers for Gastric Cancer Treatment are:
- Amit Chowdhry, MD, PhD (University of Rochester Medical Center)
- Valerie Lee, MD (Johns Hopkins University)
- Leon Pappas, MD, PhD (Dana-Farber Cancer Institute)
Any comments or questions about the summary content should be submitted to Cancer.gov through the NCI website's Email Us . Do not contact the individual Board Members with questions or comments about the summaries. Board members will not respond to individual inquiries.
Levels of Evidence
Some of the reference citations in this summary are accompanied by a level-of-evidence designation. These designations are intended to help readers assess the strength of the evidence supporting the use of specific interventions or approaches. The PDQ Adult Treatment Editorial Board uses a formal evidence ranking system in developing its level-of-evidence designations.
Permission to Use This Summary
PDQ is a registered trademark. Although the content of PDQ documents can be used freely as text, it cannot be identified as an NCI PDQ cancer information summary unless it is presented in its entirety and is regularly updated. However, an author would be permitted to write a sentence such as “NCI’s PDQ cancer information summary about breast cancer prevention states the risks succinctly: [include excerpt from the summary].”
The preferred citation for this PDQ summary is:
PDQ® Adult Treatment Editorial Board. PDQ Gastric Cancer Treatment. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at: https://www.cancer.gov/types/stomach/hp/stomach-treatment-pdq . Accessed <MM/DD/YYYY>. [PMID: 26389209]
Images in this summary are used with permission of the author(s), artist, and/or publisher for use within the PDQ summaries only. Permission to use images outside the context of PDQ information must be obtained from the owner(s) and cannot be granted by the National Cancer Institute. Information about using the illustrations in this summary, along with many other cancer-related images, is available in Visuals Online , a collection of over 2,000 scientific images.
Based on the strength of the available evidence, treatment options may be described as either “standard” or “under clinical evaluation.” These classifications should not be used as a basis for insurance reimbursement determinations. More information on insurance coverage is available on Cancer.gov on the Managing Cancer Care page.
More information about contacting us or receiving help with the Cancer.gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer.gov through the website’s Email Us .
- Patient Care & Health Information
- Diseases & Conditions
- Stomach cancer
- What is stomach cancer? A Mayo Clinic expert explains
Learn more about stomach cancer from oncologist Mohamad (Bassam) Sonbol, M.D.
I'm Dr. Bassam Sonbol, an oncologist at Mayo Clinic. In this video we'll cover the basics of stomach cancer: What is it? Who gets it? The symptoms, diagnosis, and treatment. Whether you're looking for answers for yourself or someone you love, we're here to give you the best information available. Stomach cancer, also called gastric cancer, can happen in any part of the stomach. However, in the U.S., most stomach cancers occur in the gastroesophageal junction, which is where the esophagus - the tube that carries chewed up food - meets the stomach. There are several different types of stomach cancers, but most are curable if detected at an early stage. What once was the leading cause of cancer death is now well down on the list thanks to the advancement in technology and scientific research. In fact, new cases of stomach cancers have dropped by about 1.5% every year for the last 10 years.
Stomach cancer more commonly affects older people. The average age of those diagnosed with stomach cancer is 68. Around 60% of cases occur in patients older than 65, and there is a slightly higher lifetime risk of stomach cancer in men. However, it can affect anyone. Stomach cancer tends to develop slowly over time, usually over many years. What happens is small changes occur in the DNA of the stomach cells, telling them to over multiply and then they accumulate, forming abnormal growth called tumors. There are several known risk factors that could increase your risk of developing stomach cancer, for instance, smoking doubles your risk of stomach cancer, family history of stomach cancer, infection with H. pylori, long-term stomach inflammation, gastroesophageal reflux disease, or stomach polyps. Eating a diet high in salty and smoked foods or low in fruits and vegetables can be also a risk. And there is some correlation between higher weight and risk, as well.
Stomach cancer can present itself in several different ways, such as difficulty swallowing, feeling bloated after eating, feeling full after only eating a small amount of food, heartburn, indigestion, nausea, stomach pain, unintentional weight loss, and vomiting. If you have any signs and symptoms that worry you, make an appointment with your doctor. Your doctor may investigate the more common causes of these symptoms first or refer you to a specialist, like a gastroenterologist or an oncologist, like me.
To determine if you have stomach cancer, your doctor may start with an upper endoscopy, where a tiny camera is passed through the throat and into the stomach. If your doctor finds something suspicious, they remove some tissue for a biopsy, where the cells gets sent to a lab for further analysis. Your doctor may also run some imaging tests, like CT scan or a special x-ray called a barium swallow. Identifying the extent of the cancer helps your doctor determine the best treatment. To determine the stage, they will run more tests, like blood tests, endoscopic ultrasound, CT scan, or a PET scan. In some cases, your doctor may recommend laparoscopic surgery, where the doctor inserts a special camera directly into the abdomen.
Creating a treatment plan for stomach cancer is a collaborative effort between doctors from different specialties. Our goal is to make the best treatment plan for your overall health and personal well-being. There are five main treatment options for stomach cancer: Surgery to remove all of the cancerous tissue and probably some of the healthy tissue around it. Chemotherapy, which uses drugs that journey throughout the body, destroying any cancer cells in its path. Radiation therapy, which uses high-powered beams of energy to target cancer cells. Targeted drug therapy, focusing on blocking specific weaknesses present within cancer cells. And immunotherapy, a drug treatment that helps your immune system recognize which cells are dangerous and attack them.
Finding out you have cancer can be really overwhelming and difficult. It can help to find spaces where other people understand what you're going through. Try connecting with cancer survivors online or in your community. Learning about your condition can help you make confident decisions about your care. If you'd like to learn more about stomach cancer, watch our other related videos or visit mayoclinic.org. We wish you well.
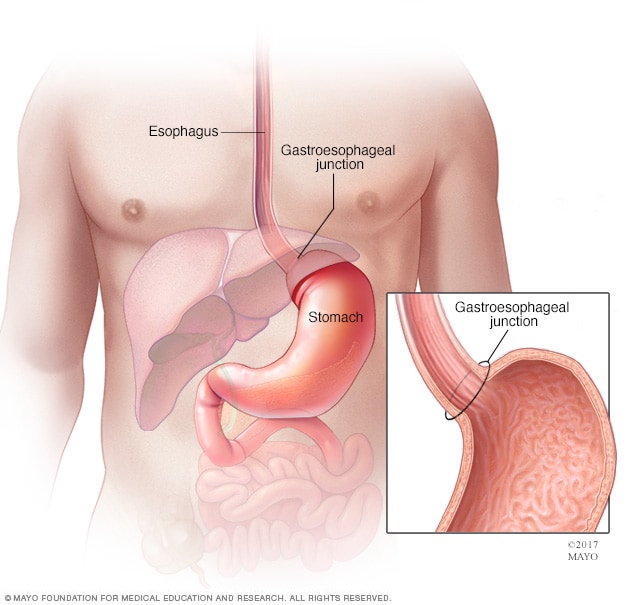
Gastroesophageal junction and stomach
The stomach is a muscular sac in the middle of the upper abdomen that helps break down and digest food. Food you eat passes down your esophagus, through the gastroesophageal junction and into the stomach.
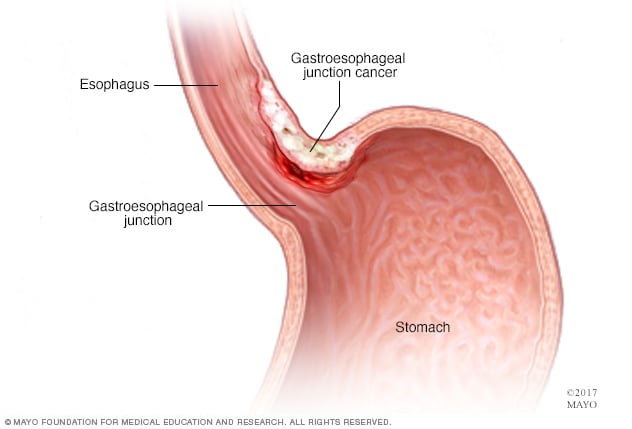
Gastroesophageal junction cancer
Cancer of the gastroesophageal junction develops in the area where the esophagus joins the top part of the stomach.
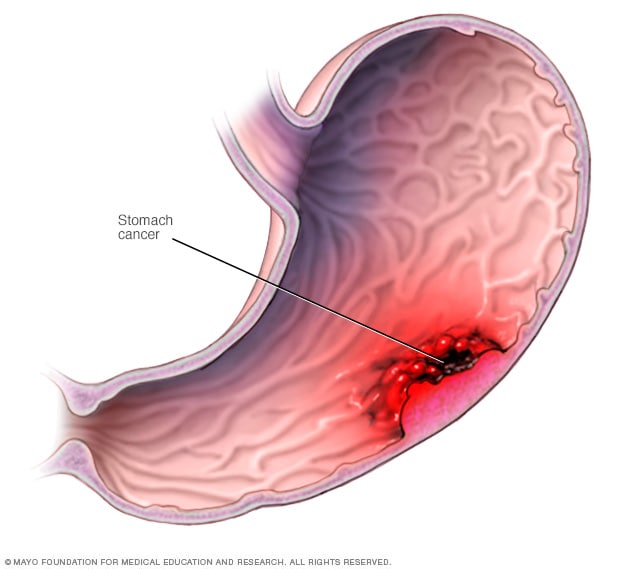
Stomach cancer most commonly begins in the cells that line the inside of the stomach.
Stomach cancer, which is also called gastric cancer, is a growth of cells that starts in the stomach. The stomach is in the upper middle part of the belly, just below the ribs. The stomach helps to break down and digest food.
Stomach cancer can happen in any part of the stomach. In most of the world, stomach cancers happen in the main part of the stomach. This part is called the stomach body.
In the United States, stomach cancer is more likely to start by the gastroesophageal junction. This is the part where the long tube that carries food you swallow meets the stomach. The tube that carries food to the stomach is called the esophagus.
Where the cancer starts in the stomach is one factor health care providers think about when making a treatment plan. Other factors might include the cancer's stage and the type of cells involved. Treatment often includes surgery to remove the stomach cancer. Other treatments may be used before and after surgery.
Stomach cancer treatment is most likely to be successful if the cancer is only in the stomach. The prognosis for people with small stomach cancers is quite good. Many can expect to be cured. Most stomach cancers are found when the disease is advanced and a cure is less likely. Stomach cancer that grows through the stomach wall or spreads to other parts of the body is harder to cure.
Products & Services
- A Book: Mayo Clinic Family Health Book, 5th Edition
- A Book: Mayo Clinic on Digestive Health
- Newsletter: Mayo Clinic Health Letter — Digital Edition
Signs and symptoms of stomach cancer may include:
- Trouble swallowing
- Feeling bloated after eating
- Feeling full after eating small amounts of food
- Not feeling hungry when you would expect to be hungry
- Indigestion
- Losing weight without trying
- Feeling very tired
- Stools that look black
Stomach cancer doesn't always cause symptoms in its early stages. When they happen, symptoms might include indigestion and pain in the upper part of the belly. Symptoms might not happen until the cancer is advanced. Later stages of stomach cancer might cause symptoms such as feeling very tired, losing weight without trying, vomiting blood and having black stools.
Stomach cancer that spreads to other parts of the body is called metastatic stomach cancer. It causes symptoms specific to where it spreads. For example, when cancer spreads to the lymph nodes it might cause lumps you can feel through the skin. Cancer that spreads to the liver might cause yellowing of the skin and whites of the eyes. If cancer spreads within the belly, it might cause fluid to fill the belly. The belly might look swollen.
When to see a doctor
If you have signs and symptoms that worry you, make an appointment with your health care provider. Many conditions can cause symptoms that are like the ones caused by stomach cancer. Your provider might test for those other causes first before testing for stomach cancer.
There is a problem with information submitted for this request. Review/update the information highlighted below and resubmit the form.
Get Mayo Clinic cancer expertise delivered to your inbox.
Subscribe for free and receive an in-depth guide to coping with cancer, plus helpful information on how to get a second opinion. You can unsubscribe at any time. Click here for an email preview.
Error Select a topic
Error Email field is required
Error Include a valid email address
To provide you with the most relevant and helpful information, and understand which information is beneficial, we may combine your email and website usage information with other information we have about you. If you are a Mayo Clinic patient, this could include protected health information. If we combine this information with your protected health information, we will treat all of that information as protected health information and will only use or disclose that information as set forth in our notice of privacy practices. You may opt-out of email communications at any time by clicking on the unsubscribe link in the e-mail.
Thank you for subscribing
Your in-depth coping with cancer guide will be in your inbox shortly. You will also receive emails from Mayo Clinic on the latest about cancer news, research, and care.
If you don’t receive our email within 5 minutes, check your SPAM folder, then contact us at [email protected] .
Sorry something went wrong with your subscription
Please, try again in a couple of minutes
It's not clear what causes stomach cancer. Experts believe most stomach cancers start when something hurts the inside lining of the stomach. Examples include having an infection in the stomach, having long-standing acid reflux and eating a lot of salty foods. Not everyone with these risk factors gets stomach cancer, though. So more research is needed to find out exactly what causes it.
Stomach cancer begins when something hurts cells in the inner lining of the stomach. It causes the cells to develop changes in their DNA. A cell's DNA holds the instructions that tell a cell what to do. The changes tell the cells to multiply quickly. The cells can go on living when healthy cells would die as part of their natural lifecycle. This causes a lot of extra cells in the stomach. The cells can form a mass called a tumor.
Cancer cells in the stomach can invade and destroy healthy body tissue. They might start to grow deeper into the wall of the stomach. In time, cancer cells can break away and spread to other parts of the body. When cancer cells spread to another part of the body it's called metastasis.
Types of stomach cancer
The type of stomach cancer you have is based on the type of cell where your cancer began. Examples of stomach cancer types include:
- Adenocarcinoma. Adenocarcinoma stomach cancer starts in cells that produce mucus. This is the most common type of stomach cancer. Nearly all cancers that start in the stomach are adenocarcinoma stomach cancers.
- Gastrointestinal stromal tumors (GIST). GIST starts in special nerve cells that are found in the wall of the stomach and other digestive organs. GIST is a type of soft tissue sarcoma.
- Carcinoid tumors. Carcinoid tumors are cancers that start in the neuroendocrine cells. Neuroendocrine cells are found in many places in the body. They do some nerve cell functions and some of the work of cells that make hormones. Carcinoid tumors are a type of neuroendocrine tumor.
- Lymphoma. Lymphoma is a cancer that starts in immune system cells. The body's immune system fights germs. Lymphoma can sometimes start in the stomach if the body sends immune system cells to the stomach. This might happen if the body is trying to fight off an infection. Most lymphomas that start in the stomach are a type of non-Hodgkin's lymphoma.
Risk factors
Factors that increase the risk of stomach cancer include:
- Ongoing problems with stomach acid backing up into the esophagus, which is called gastroesophageal reflux disease
- A diet high in salty and smoked foods
- A diet low in fruits and vegetables
- Infection in the stomach caused by a germ called Helicobacter pylori
- Swelling and irritation of the inside of the stomach, which is called gastritis
- Growths of noncancerous cells in the stomach, called polyps
- Family history of stomach cancer
- Family history of genetic syndromes that increase the risk of stomach cancer and other cancers, such as hereditary diffuse gastric cancer, Lynch syndrome, juvenile polyposis syndrome, Peutz-Jeghers syndrome and familial adenomatous polyposis
To lower the risk of stomach cancer, you can:
- Eat plenty of fruits and vegetables. Try to include fruits and vegetables in your diet each day. Choose a variety of colorful fruits and vegetables.
- Reduce the amount of salty and smoked foods you eat. Protect your stomach by limiting these foods.
- Stop smoking. If you smoke, quit. If you don't smoke, don't start. Smoking increases your risk of stomach cancer and many other types of cancer. Quitting smoking can be very hard, so ask your health care provider for help.
- Tell your health care provider if stomach cancer runs in your family. People with a strong family history of stomach cancer might have stomach cancer screening. Screening tests can detect stomach cancer before it causes symptoms.
Stomach cancer care at Mayo Clinic
Living with stomach cancer?
Connect with others like you for support and answers to your questions in the Cancer support group on Mayo Clinic Connect, a patient community.
Cancer Discussions

87 Replies Sat, May 25, 2024

9 Replies Sat, May 25, 2024

37 Replies Sat, May 25, 2024
- AskMayoExpert. Gastric cancer (adult). Mayo Clinic; 2020.
- Gastric cancer. National Comprehensive Cancer Network. https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1434. Accessed July 22, 2022.
- Niederhuber JE, et al., eds. Cancer of the stomach. In: Abeloff's Clinical Oncology. 6th ed. Elsevier; 2020. https://www.clinicalkey.com. Accessed July 22, 2022.
- Gastric cancer treatment (PDQ). National Cancer Institute. https://www.cancer.gov/types/stomach/patient/stomach-treatment-pdq. Accessed July 22, 2022.
- Gastric (stomach) cancer prevention (PDQ). National Cancer Institute. https://www.cancer.gov/types/stomach/patient/stomach-prevention-pdq. Accessed July 22, 2022.
- Palliative care. National Comprehensive Cancer Network. https://www.nccn.org/guidelines/guidelines-detail?category=3&id=1454. Accessed July 22, 2022.
- Odze RD, et al., eds. Epithelial neoplasms of the stomach. In: Surgical Pathology of the GI Tract, Liver, Biliary Tract and Pancreas. 4th ed. Elsevier; 2023. https://www.clinicalkey.com. Accessed Aug. 5, 2022.
- Mansfield PF. Clinical features, diagnosis and staging of gastric cancer. https://www.uptodate.com/contents/search. Accessed Aug. 5, 2022.
- Andreas A, et al., eds. The stomach. In: Grainger & Allison's Diagnostic Radiology: A Textbook of Medical Imaging. 7th ed. Elsevier; 2021. https://www.clinicalkey.com. Accessed Aug. 5, 2022.
- Xia JY, et al. Advances in screening and detection of gastric cancer. Journal of Surgical Oncology. 2022; doi:10.1002/jso.26844.
- Best hospitals for gastroenterology and GI surgery. U.S. News & World Report. https://health.usnews.com/best-hospitals/rankings/gastroenterology-and-gi-surgery. Accessed Aug. 2, 2022.
- Best hospitals for cancer. U.S. News & World Report. https://health.usnews.com/best-hospitals/rankings/cancer. Accessed Sept. 9, 2022.
- Warner KJ. Allscripts EPSi. Mayo Clinic. Feb. 12, 2020.
- Stomach cancer FAQs
Associated Procedures
- Barium enema
- Chemotherapy
- Endoscopic ultrasound
- Needle biopsy
- Palliative care
- Positron emission tomography scan
- Radiation therapy
- Upper endoscopy
News from Mayo Clinic
- Mayo Clinic Minute: New chemotherapy approach for treating stomach cancer May 23, 2024, 04:30 p.m. CDT
- New heated drug baths provide hope for patients with stomach cancer April 04, 2024, 05:15 p.m. CDT
- Mayo Clinic Minute: Stomach cancer concerns Nov. 10, 2023, 05:00 p.m. CDT
Mayo Clinic in Rochester, Minnesota, Mayo Clinic in Jacksonville, Florida, and Mayo Clinic in Phoenix/Scottsdale, Arizona, have been recognized among the top Cancer hospitals in the nation for 2023-2024 by U.S. News & World Report.
- Symptoms & causes
- Diagnosis & treatment
- Doctors & departments
- Care at Mayo Clinic
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
Your gift holds great power – donate today!
Make your tax-deductible gift and be a part of the cutting-edge research and care that's changing medicine.
- What is Gastric Cancer?
- Symptoms and Diagnosis
- Development and Stages
- Biomarkers and Gastric Cancer
- Treatment Options
- Research Initiatives
- Gastric Cancer Registry
- Early Stage Research Grants
- Research Scholar Award
- Clinical Trial Finder
- Published Gastric Cancer Research
- Resources and Support
- Online Community
- Treatment and Survivorship Care Planner
- Latest News
- Eating and Healing
- Watch Episodes
- Printable Recipes
- Giving Options
- Memorials and Tributes
- Sponsor Opportunities
- Board of Directors
- Sponsors & Partners
- Events & Campaigns
- Financial Reports
CONNECT WITH FELLOW PATIENTS AND CAREGIVERS
Remember, you are not alone. Ask questions, share advice and find support in a confidential forum that enables candid conversations among people who share the same journey.
MEET YOUR COMMUNITY
JOIN YOUR COMMUNITY ONLINE
When it comes to your healthcare journey, fellow patients and caregivers are invaluable yet often untapped resources.
We are proud to partner with Smart Patients to offer a secure online peer-to-peer support community where people affected by gastric cancer can share advice, resources, and support.
Your well-being is our priority. Our forum is a confidential space, limited to those who have been specifically impacted by gastric cancer. Here, you’ll meet individuals who understand the unique challenges of this cancer journey and are ready to share their insights with compassion.
Smart Patients is a leader in creating online networks of engaged patients and families facing a variety of illnesses.
YOUR COMMUNITY IS WAITING TO WELCOME YOU. START A CONVERSATION TODAY.
SIGN UP NOW
“This group has already made me feel at peace. It’s always good to be armed with information and medical professionals, but it’s immeasurable to be armed with support.”
“Smart Patients is much more than a support network. It’s a study group. It’s a knowledge repository. It’s an exercise in participatory medicine.”
“Some of these health issues can cause a feeling of isolation and it’s great to hear that there are supportive and kind people out there who ‘get it.’”
JOIN THE CONVERSATION
- GP practice services
- Health advice
- Health research
- Medical professionals
- Health topics
Advice and clinical information on a wide variety of healthcare topics.
All health topics
Latest features
Allergies, blood & immune system
Bones, joints and muscles
Brain and nerves
Chest and lungs
Children's health
Cosmetic surgery
Digestive health
Ear, nose and throat
General health & lifestyle
Heart health and blood vessels
Kidney & urinary tract
Men's health
Mental health
Oral and dental care
Senior health
Sexual health
Signs and symptoms
Skin, nail and hair health
Travel and vaccinations
Treatment and medication
Women's health
Healthy living
Expert insight and opinion on nutrition, physical and mental health.
Exercise and physical activity
Healthy eating
Healthy relationships
Managing harmful habits
Mental wellbeing
Relaxation and sleep
Managing conditions
From ACE inhibitors for high blood pressure, to steroids for eczema, find out what options are available, how they work and the possible side effects.
Featured conditions
ADHD in children
Crohn's disease
Endometriosis
Fibromyalgia
Gastroenteritis
Irritable bowel syndrome
Polycystic ovary syndrome
Scarlet fever
Tonsillitis
Vaginal thrush
Health conditions A-Z
Medicine information
Information and fact sheets for patients and professionals. Find out side effects, medicine names, dosages and uses.
All medicines A-Z
Allergy medicines
Analgesics and pain medication
Anti-inflammatory medicines
Breathing treatment and respiratory care
Cancer treatment and drugs
Contraceptive medicines
Diabetes medicines
ENT and mouth care
Eye care medicine
Gastrointestinal treatment
Genitourinary medicine
Heart disease treatment and prevention
Hormonal imbalance treatment
Hormone deficiency treatment
Immunosuppressive drugs
Infection treatment medicine
Kidney conditions treatments
Muscle, bone and joint pain treatment
Nausea medicine and vomiting treatment
Nervous system drugs
Reproductive health
Skin conditions treatments
Substance abuse treatment
Vaccines and immunisation
Vitamin and mineral supplements
Tests & investigations
Information and guidance about tests and an easy, fast and accurate symptom checker.
About tests & investigations
Symptom checker
Blood tests
BMI calculator
Pregnancy due date calculator
General signs and symptoms
Patient health questionnaire
Generalised anxiety disorder assessment
Medical professional hub
Information and tools written by clinicians for medical professionals, and training resources provided by FourteenFish.
Content for medical professionals
FourteenFish training
Professional articles
Evidence-based professional reference pages authored by our clinical team for the use of medical professionals.
View all professional articles A-Z
Actinic keratosis
Bronchiolitis
Molluscum contagiosum
Obesity in adults
Osmolality, osmolarity, and fluid homeostasis
Recurrent abdominal pain in children
Medical tools and resources
Clinical tools for medical professional use.
All medical tools and resources
Stomach cancer
Gastric cancer.
Peer reviewed by Dr Hayley Willacy, FRCGP Last updated by Dr Doug McKechnie, MRCGP Last updated 10 Feb 2023
Meets Patient’s editorial guidelines
Although stomach (gastric) cancer is common worldwide, it is not so common in the UK. Most cases occur in people over the age of 55. If stomach cancer is diagnosed at an early stage, there is a good chance of a cure. In general, the more advanced the cancer (the more it has grown and spread), the less chance that treatment will be curative. However, treatment can often slow the progress of the cancer.
In this article :
What is stomach cancer, what are the types of stomach cancer, what is the stomach, what causes stomach cancer, stomach cancer symptoms, how is stomach cancer diagnosed and assessed, how is stomach cancer treated, the aim of stomach cancer treatment, can stomach cancer be cured, stomach cancer prevention.
Continue reading below
Stomach cancer is sometimes called gastric cancer. Worldwide it is one of the most common cancers. It is common in Japan and China but is less common in the UK. About 5,000 people develop stomach cancer each year in the UK. Stomach cancer is more common in men than in women and tends to occur mainly in older people. Most people who develop stomach cancer are over the age of 55.
Adenocarcinoma of the stomach
In most cases, stomach cancer begins from a cell which is on the inside lining of the stomach (the mucosa). This type of stomach cancer is called adenocarcinoma of the stomach. As the cancer cells multiply:
The tumour may invade deeper into the wall of the stomach. In time, it may pass through the wall of the stomach and invade nearby organs such as the pancreas or liver.
The tumour may spread up or down the stomach into the gullet (oesophagus) or small intestine.
Some cells may break off into the lymph channels or bloodstream. The cancer may then spread to nearby lymph nodes or spread to other areas of the body (metastasise).
Other types of stomach cancer
There are some less common and rare types of stomach cancer which include:
Lymphomas. These are cancers which arise from the lymphatic tissue (part of the immune system) within the wall of the stomach.
Sarcomas. These are cancers which arise from the muscle or connective tissue within the wall of the stomach.
Carcinoid cancers. These are cancers which arise from cells in the stomach lining which make hormones.
The rest of this leaflet only discusses adenocarcinoma of the stomach.
See the separate leaflet called Cancer for more general information about cancer .
The upper gut
To understand stomach cancer, it helps to know about the normal structure and function of the stomach.
The stomach is in the upper tummy (abdomen). It is part of the gut (gastrointestinal tract). It lies in the upper part of the abdomen, just below the ribs. When we eat, food passes down the gullet (oesophagus) into the stomach.
The stomach is part of the digestive system and makes acid and some chemicals (enzymes) which help to digest food. The muscles in the wall of the stomach tighten (contract) to mix up the food with the acid and enzymes.
Food then passes into the first part of the small intestine (the duodenum). Here food mixes with more enzymes which come from the pancreas and lining of the gut. The enzymes break down (digest) the food.
Digested food is then absorbed into the body from the small intestine.
A cancerous (malignant) tumour starts from one abnormal cell. The exact reason why a cell becomes cancerous is unclear. It is thought that something damages or alters certain genes in the cell. This makes the cell abnormal and multiply out of control. ( See the separate leaflet called Cancer for more details .)
Many people develop stomach cancer for no apparent reason. However, certain risk factors increase the chance that stomach cancer may develop. These include:
Ageing. Stomach cancer is more common in older people. Most cases are in people over the age of 55.
Having a type of anaemia called pernicious anaemia, which causes a lack of vitamin B12, can very slightly increase your risk of stomach cancer.
Diet is probably a factor:
Countries such as Japan, where people eat a lot of salt, and pickled and smoked foods, have a high rate of stomach cancer.
Eating a lot of fruit and green vegetables can reduce the risk.
Smokers have a higher rate of stomach cancer compared with people who do not smoke.
Long-term infection of the stomach lining with a germ (bacterium) called Helicobacter pylori ( H. pylori ) seems to lead to a slightly higher risk of stomach cancer. (This infection is very common in the UK, and most people with H. pylori infection do not develop stomach cancer. See the separate leaflet called Helicobacter Pylori and Stomach Pain for more details .)
Gender. Stomach cancer is twice as common in men as it is in women.
If you have had part of your stomach removed in the past for any reason. For example, to treat a stomach ulcer or some other condition.
Family history. For some cases, stomach cancer may run in the family. However, most cases of stomach cancer do not run in families and are not inherited.
Blood group A. People who have this blood group have a slightly higher risk.
When a stomach (gastric) cancer first develops and is small, it usually causes no symptoms. Some do not cause symptoms until they are quite advanced. Initial symptoms may include:
Pain or discomfort in the upper tummy (abdomen), especially after eating.
Indigestion . ( Note : most people who have indigestion do not have stomach cancer.)
Feeling sick, and being off food. Some people have a sense of fullness after eating.
Weight loss and/or loss of appetite.
You may pass blood out with your stools (faeces). This usually presents as black faeces (called melaena) or dark blood rather than bright red bleeding - which is more unusual with stomach cancer - and implies very serious bleeding in the stomach or bowel.
As the cancer grows in the stomach, symptoms may become worse and may include:
The same symptoms as above, but more severe.
Feeling generally unwell and more tired than usual.
Becoming anaemic if the tumour regularly bleeds. This can cause you to become more tired than usual.
The cancer growing very large and causing a blockage to food and drink.
If the cancer spreads to other parts of the body, various other symptoms can develop.
Note : all the above symptoms can be due to other conditions, so tests are needed to confirm stomach cancer.
Editor’s note
Dr Krishna Vakharia , 16th October 2023
The National Institute for Health and Care Excellence (NICE) has recommended that a person should receive a diagnosis or ruling out of cancer within 28 days of being referred urgently by their GP for suspected cancer.
Initial assessment and gastroscopy (endoscopy)
If a doctor suspects that you may have stomach (gastric) cancer, he or she may examine you. The examination is often normal, especially if the cancer is at an early stage. Therefore, a gastroscopy is usually arranged. A gastroscope (endoscope) is a thin, flexible, telescope. It is passed through the mouth, into the gullet (oesophagus) and down towards the stomach and the first part of the small intestine (the duodenum). The endoscope contains fibre-optic channels which allow light to shine down so the doctor or nurse can see inside your stomach and duodenum. ( See the separate leaflet called Gastroscopy (Endoscopy) for more details .)
Biopsy - to confirm the diagnosis
When a small sample of tissue is removed from a part of the body, the procedure is called a biopsy . The sample is then examined under the microscope to look for abnormal cells. When you have a gastroscopy, if anything abnormal is seen, the doctor or nurse can take a biopsy. This is done by passing a thin grabbing instrument down a side channel of the gastroscope. It can take two weeks for the biopsy results.
Assessing the extent and spread
If you are confirmed to have stomach cancer, further tests may be done to assess if it has spread. For example, a barium meal X-ray , a computerised tomography (CT) scan , a magnetic resonance imaging (MRI) scan , an ultrasound scan , laparoscopy or other tests such as blood tests. This assessment is called staging of the cancer.
The aim of staging is to find out:
How much the tumour in the stomach has grown, and whether it has grown partially or fully through the wall of the stomach.
Whether the cancer has spread to local lymph nodes.
Whether the cancer has spread to other areas of the body (metastasised).
By finding out the stage of the cancer it helps doctors to advise on the best treatment options. It also gives a reasonable indication of outlook (prognosis). ( See the separate leaflet called Stages of Cancer for more details .)
Treatment options which may be considered include surgery, chemotherapy (and sometimes radiotherapy). The treatment advised for each case depends on various factors, such as:
How large the cancer is.
Whether it has spread (the stage of the cancer).
Your general health.
Surgery to remove the tumour may be curative if the cancer is in an early stage. The common operation is to cut out the affected part of the stomach. Sometimes the whole of the stomach is removed. Sometimes this is done laparoscopically (keyhole surgery). Even if the cancer is advanced and a cure is not possible, some surgical techniques may still have a place to ease symptoms. For example, a blockage may be eased by removing part of the stomach, or by using laser surgery or by a bypass operation.
Chemotherapy
Chemotherapy is a treatment of cancer by using anti-cancer medicines which kill cancer cells or stop them from multiplying. (See the separate leaflet called Chemotherapy for more details.) When chemotherapy is used in addition to surgery it is known as adjuvant chemotherapy. For example, following surgery you may be given a course of chemotherapy. This aims to kill any cancer cells which may have spread away from the primary tumour. Sometimes, chemotherapy is given before surgery, to shrink a large tumour so that surgery is easier - this is known as neoadjuvant chemotherapy.
Radiotherapy
Radiotherapy is a treatment which uses high-energy beams of radiation which are focused on cancerous tissue. This kills cancer cells, or stops cancer cells from multiplying. ( See the separate leaflet called Radiotherapy for more details .) Radiotherapy is not commonly used to treat stomach cancer. It is sometimes used to reduce the size of a particular part of the cancer which is causing symptoms.
Chemoradiotherapy
In some cases, a combination of chemotherapy and radiotherapy - shortened to chemoradiotherapy - is offered, often as an addition to surgery. If you need to have this, it is usually done around the time of the operation.
Food and diet
It is important to make sure you get enough nutrition from your food and you will probably be asked to see a dietician to make sure you are on the best possible diet.
You should have a full discussion with a specialist who knows your case. They will be able to give the pros and cons, likely success rate, possible side-effects, and other details about the various possible treatment options for your type of cancer.
You should also discuss with your specialist about the aims of treatment in your situation.
To cure cancer
Treatment may aim to cure the cancer. Some stomach (gastric) cancers can be cured, particularly if they are treated in the early stages of the disease. (Doctors tend to use the word remission rather than the word cured. Remission means there is no evidence of cancer following treatment. If you are in remission, you may be cured. However, in some cases a cancer returns months or years later. This is why doctors are sometimes reluctant to use the word cured.)
To control cancer
Treatment may aim to control the cancer. If a cure is not realistic, with treatment it is often possible to limit the growth or spread of the cancer so that it progresses less rapidly. This may keep you free of symptoms for some time.
To ease symptoms
Treatment may aim to ease symptoms. If a cure is not possible, treatments may be used to reduce the size of a cancer, which may ease symptoms such as pain. If a cancer is advanced, you may require treatments such as:
Food supplements.
Painkillers.
Other techniques to help keep you free of pain or other symptoms.
Without treatment, a stomach (gastric) cancer is likely to get larger, and spread to other parts of the body. If it is diagnosed and treated at an early stage (before growing through the wall of the stomach or spreading to lymph nodes or other areas of the body) then there is a good chance of a cure with surgery. Unfortunately, most cases in the UK are not diagnosed at an early stage. Stomach cancer often causes no, or few, symptoms in the early stages. When it does cause symptoms, they can be easily mistaken for other more common stomach conditions at first.
If the cancer is diagnosed when it has grown through the wall of the stomach, or spread to other parts of the body, a cure is less likely. However, treatment can often slow down the progression of the cancer, using chemotherapy medicines.
The treatment of cancer is a developing area of medicine. New treatments continue to be developed and the information on outlook (prognosis) above is very general. The specialist who knows your case can give more accurate information about your particular outlook, and how well your type and stage of cancer is likely to respond to treatment.
We don't have a way to prevent stomach cancer completely.
However, there are some things which can help to reduce the risk of it happening, such as:
Stopping, or avoiding, smoking. Smoking increases the risk of stomach cancer, and many other types of cancer.
Eating a healthy diet with plenty of fresh fruit and vegetables, and whole-grain cereals.
Maintaining a healthy weight. Obesity has been linked with a higher risk of stomach cancer (and other cancers), so losing weight if overweight or obese may reduce the risk.
Treating Helicobacter pylori infection . Helicobacter pylori infection is linked with an increased risk of stomach cancer, although the risk is small. Treating Helicobacter pylori reduces this risk. Currently, in the UK, we only test for Helicobacter pylori in people with symptoms of stomach problems (see the Helicobacter pylori leaflet for more information). Testing people without symptoms, or 'screening', for Helicobacter pylori , is probably not an effective way to prevent stomach cancer in the UK, but some people have suggested it might be beneficial in countries where stomach cancer is more common.
Further reading and references
- Stomach cancer incidence statistics ; Cancer Research UK
- Gastric Cancer Treatment Patient Version ; PDQ Cancer Information Summaries. National Cancer Institute (US); 2002-2017 Apr 27
- Oesophago-gastric cancer: assessment and management in adults ; NICE Guidance (Jan 2018)
- Lordick F, Carneiro F, Cascinu S, et al ; Gastric cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022 Oct;33(10):1005-1020. doi: 10.1016/j.annonc.2022.07.004. Epub 2022 Jul 29.
Article history
The information on this page is written and peer reviewed by qualified clinicians.
Next review due: 9 Feb 2028
10 feb 2023 | latest version.
Last updated by
Peer reviewed by

Feeling unwell?
Assess your symptoms online for free
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review article
- Published: 07 November 2022
Current developments in gastric cancer: from molecular profiling to treatment strategy
- Maria Alsina ORCID: orcid.org/0000-0003-4835-7159 1 , 2 , 3 ,
- Virginia Arrazubi 2 , 3 ,
- Marc Diez ORCID: orcid.org/0000-0002-9473-0391 1 , 4 &
- Josep Tabernero ORCID: orcid.org/0000-0002-2495-8139 1 , 4
Nature Reviews Gastroenterology & Hepatology volume 20 , pages 155–170 ( 2023 ) Cite this article
7952 Accesses
73 Citations
71 Altmetric
Metrics details
- Gastric cancer
- Molecular medicine
Gastric cancer and gastro-oesophageal junction cancer represent a global health-care challenge. Despite the efficacy of improved chemotherapy and surgical options, these patients still have a poor prognosis. In advanced disease, only trastuzumab and some immune checkpoint inhibitors, such as nivolumab and pembrolizumab in addition to chemotherapy, have demonstrated consistent and reliable efficacy in patients with HER2-positive and PDL1-positive tumours, respectively. In this Review, we discuss the intrinsic characteristics of gastric and gastro-oesophageal cancer from the molecular and clinical perspectives and provide a comprehensive review of previously reported and ongoing phase II and III clinical trials with targeted agents and immunotherapy in advanced and localized settings. Finally, we suggest alternative strategies to help overcome current challenges in precision medicine and to improve outcomes for these patients.
The spatial and temporal heterogeneity features of gastric and gastro-oesophageal tumours have jeopardized the success of most phase II and III clinical trials with targeted treatments.
Current biomarkers for treatment decisions in patients with gastric and gastro-oesophageal cancer include testing for HER2 overexpression and amplification, PDL1 combined positive score expression and microsatellite instability-high status.
Chemotherapy drugs of reduced toxicity, together with some molecularly driven targeted therapies, have been shown to be particularly valuable for sequential treatment strategies in optimizing the survival of patients with gastric and gastro-oesophageal cancer.
National strategies with massive molecular screening of patients with gastric and gastro-oesophageal cancer have emerged as promising approaches to detect those candidates who could benefit from targeted treatments.
This is a preview of subscription content, access via your institution

Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
195,33 € per year
only 16,28 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
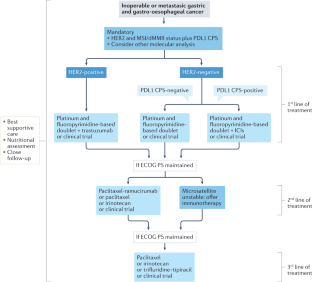
Similar content being viewed by others

Biomarker-targeted therapies for advanced-stage gastric and gastro-oesophageal junction cancers: an emerging paradigm
Resistance to immune checkpoint inhibitors in advanced gastro-oesophageal cancers
Early stage gastric adenocarcinoma: clinical and molecular landscapes
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. https://doi.org/10.3322/caac.21660 (2021).
Article PubMed Google Scholar
Bosman, F. T., Carneiro, F., Hruban, R. H. & Theise, N. D. WHO classification of tumours of the digestive system: WHO Classification of Tumours 4th edn. Vol. 3 (IARC, 2010).
Bass, A. J. et al. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 513 , 202–209 (2014).
Article Google Scholar
Cristescu, R. et al. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat. Med. 21 , 449–456 (2015).
Article CAS PubMed Google Scholar
Thun, M. J., Wild, C. P. & Colditz, G. in Schottenfeld Fraumeni Cancer Epidemiology and Prevention 4th edn (eds Thun, M. J., Linet, M. S., Cerhan, J. R., Haiman, C. A. & Schottenfeld, D.) 1193–1204 (Oxford Univ. Press, 2017).
The Cancer Genome Atlas Research Network. Integrated genomic characterization of oesophageal carcinoma. Nature 541 , 169–175 (2017).
Article PubMed Central Google Scholar
Lordick, F. et al. Gastric cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. https://doi.org/10.1016/J.ANNONC.2022.07.004 (2022).
American Cancer Society. Cancer facts & figures 2021 (American Cancer Society, 2021).
Davidson, M. et al. Survival in advanced esophagogastric adenocarcinoma improves with use of multiple lines of therapy: results from an analysis of more than 500 patients. Clin Colorectal Cancer 17 , 223–230 (2018).
Fanotto, V. et al. Outcomes of advanced gastric cancer patients treated with at least three lines of systemic chemotherapy. Oncologist 22 , 1463–1469 (2017).
Article PubMed PubMed Central Google Scholar
Cafferkey, C. et al. Survival in advanced oesophagogastric adenocarcinoma (OGA) improves with the use of multiple lines of therapy: results from an analysis of over 500 patients (pts) [abstract 642P]. Ann. Oncol. 28 (Suppl. 5), v219 (2017).
Hess, L. M. et al. Chemotherapy treatment patterns, costs, and outcomes of patients with gastric cancer in the United States: a retrospective analysis of electronic medical record (EMR) and administrative claims data. Gastric Cancer 19 , 607–615 (2016).
Deng, N. et al. A comprehensive survey of genomic alterations in gastric cancer reveals systematic patterns of molecular exclusivity and co-occurrence among distinct therapeutic targets. Gut 61 , 673–684 (2012).
Dulak, A. M. et al. Gastrointestinal adenocarcinomas of the esophagus, stomach, and colon exhibit distinct patterns of genome instability and oncogenesis. Cancer Res. 72 , 4383–4393 (2012).
Article CAS PubMed PubMed Central Google Scholar
Sohn, B. H. et al. Clinical significance of four molecular subtypes of gastric cancer identified by The Cancer Genome Atlas project. Clin. Cancer Res. 23 , 4441–4449 (2017).
Janjigian, Y. Y. et al. Genetic predictors of response to systemic therapy in esophagogastric cancer. Cancer Discov. 8 , 49–58 (2018).
Suh, Y. S. et al. Comprehensive molecular characterization of adenocarcinoma of the gastroesophageal junction between esophageal and gastric adenocarcinomas. Ann. Surg. 275 , 706–717 (2022).
Tabernero, J. et al. End-of-study analysis from JACOB: A phase III study of pertuzumab (P) + trastuzumab (H) and chemotherapy (CT) in HER2-positive metastatic gastric or gastro-esophageal junction cancer (mGC/GEJC) [abstract 1423MO]. Ann. Oncol. 31 (Suppl. 4), S900–S901 (2020).
Satoh, T. et al. Lapatinib plus paclitaxel versus paclitaxel alone in the second-line treatment of HER2-amplified advanced gastric cancer in Asian populations: TyTAN–a randomized, phase III study. J. Clin. Oncol. 32 , 2039–2049 (2014).
Hecht, J. R. et al. Lapatinib in combination with capecitabine plus oxaliplatin in human epidermal growth factor receptor 2-positive advanced or metastatic gastric, esophageal, or gastroesophageal adenocarcinoma: TRIO-013/LOGiC–a randomized phase III trial. J. Clin. Oncol. 34 , 443–451 (2016).
Thuss-Patience, P. C. et al. Trastuzumab emtansine versus taxane use for previously treated HER2-positive locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma (GATSBY): an international randomised, open-label, adaptive, phase 2/3 study. Lancet Oncol. 18 , 640–653 (2017).
Waddell, T. et al. Epirubicin, oxaliplatin, and capecitabine with or without panitumumab for patients with previously untreated advanced oesophagogastric cancer (REAL3): a randomised, open-label phase 3 trial. Lancet Oncol. 14 , 481–489 (2013).
Lordick, F. et al. Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open-label phase 3 trial. Lancet Oncol. 14 , 490–499 (2013).
Dutton, S. J. et al. Gefitinib for oesophageal cancer progressing after chemotherapy (COG): a phase 3, multicentre, double-blind, placebo-controlled randomised trial. Lancet Oncol. 15 , 894–904 (2014).
Catenacci, D. V. T. et al. Rilotumumab plus epirubicin, cisplatin, and capecitabine as first-line therapy in advanced MET-positive gastric or gastro-oesophageal junction cancer (RILOMET-1): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 18 , 1467–1482 (2017).
Shah, M. A. et al. Effect of fluorouracil, leucovorin, and oxaliplatin with or without onartuzumab in HER2-negative, MET-positive gastroesophageal adenocarcinoma: the METGastric Randomized Clinical Trial. JAMA Oncol. 3 , 620–627 (2017).
Alsina, M., Gullo, I. & Carneiro, F. Intratumoral heterogeneity in gastric cancer: a new challenge to face. Ann. Oncol. 28 , 912–913 (2017).
Nakamura, Y., Kawazoe, A., Lordick, F., Janjigian, Y. Y. & Shitara, K. Biomarker-targeted therapies for advanced-stage gastric and gastro-oesophageal junction cancers: an emerging paradigm. Nat. Rev. Clin. Oncol. 18 , 473–487 (2021).
Pectasides, E. et al. Genomic heterogeneity as a barrier to precision medicine in gastroesophageal adenocarcinoma. Cancer Discov. 8 , 37–48 (2018).
Gullo, I., Carneiro, F., Oliveira, C. & Almeida, G. M. Heterogeneity in gastric cancer: from pure morphology to molecular classifications. Pathobiology 85 , 50–63 (2018).
Catenacci, D. V. T. et al. Personalized antibodies for gastroesophageal adenocarcinoma (PANGEA): a phase II study evaluating an individualized treatment strategy for metastatic disease. Cancer Discov. 11 , 308–325 (2021).
Nakamura, Y. et al. Emergence of concurrent multiple EGFR mutations and MET amplification in a patient with EGFR-amplified advanced gastric cancer treated with cetuximab. JCO Precis. Oncol. 4 , 1407–1413 (2020).
Parikh, A. R. et al. Liquid versus tissue biopsy for detecting acquired resistance and tumor heterogeneity in gastrointestinal cancers. Nat. Med. 25 , 1415–1421 (2019).
Marshall, B. J. & Warren, J. R. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet 323 , 1311–1315 (1984).
Engstrand, L. & Graham, D. Y. Microbiome and gastric cancer. Dig. Dis. Sci. 65 , 865–873 (2020).
Yang, J., Zhou, X., Liu, X., Ling, Z. & Ji, F. Role of the gastric microbiome in gastric cancer: from carcinogenesis to treatment. Front. Microbiol. 12 , 641322 (2021).
Wu, F. et al. Oral and gastric microbiome in relation to gastric intestinal metaplasia. Int. J. Cancer 150 , 928–940 (2022).
Ferreira, R. M. et al. Gastric microbial community profiling reveals a dysbiotic cancer-associated microbiota. Gut 67 , 226–236 (2018).
Al-Batran, S. E. et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet 393 , 1948–1957 (2019).
Cunningham, D. et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N. Engl. J. Med. 355 , 11–20 (2006).
Smyth, E. C. et al. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 27 (Suppl. 5), v38–v49 (2016).
Thuss-Patience, P. C. et al. Survival advantage for irinotecan versus best supportive care as second-line chemotherapy in gastric cancer – a randomised phase III study of the Arbeitsgemeinschaft Internistische Onkologie (AIO). Eur. J. Cancer 47 , 2306–2314 (2011).
Ford, H. E. R. et al. Docetaxel versus active symptom control for refractory oesophagogastric adenocarcinoma (COUGAR-02): an open-label, phase 3 randomised controlled trial. Lancet Oncol. 15 , 78–86 (2014).
Iizumi, S., Takashima, A., Sakamaki, K., Morita, S. & Boku, N. Survival impact of post-progression chemotherapy in advanced gastric cancer: systematic review and meta-analysis. Cancer Chemother. Pharmacol. 81 , 981–989 (2018).
Cunningham, D. et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N. Engl. J. Med. 358 , 36–46 (2008).
Al-Batran, S. E. et al. Phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil, leucovorin plus either oxaliplatin or cisplatin: a study of the Arbeitsgemeinschaft Internistische Onkologie. J. Clin. Oncol. 26 , 1435–1442 (2008).
Hall, P. S. et al. Efficacy of reduced-intensity chemotherapy with oxaliplatin and capecitabine on quality of life and cancer control among older and frail patients with advanced gastroesophageal cancer: the GO2 phase 3 randomized clinical trial. JAMA Oncol. 7 , 869–877 (2021).
Bang, Y.-J. et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 376 , 687–697 (2010).
Janjigian, Y. Y. et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet 398 , 27–40 (2021).
Sun, J. M. et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet 398 , 759–771 (2021).
Tabernero, J. et al. Pertuzumab plus trastuzumab and chemotherapy for HER2-positive metastatic gastric or gastro-oesophageal junction cancer (JACOB): final analysis of a double-blind, randomised, placebo-controlled phase 3 study. Lancet Oncol. 19 , 1372–1384 (2018).
Ohtsu, A. et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J. Clin. Oncol. 29 , 3968–3976 (2011).
Fuchs, C. S. et al. Ramucirumab with cisplatin and fluoropyrimidine as first-line therapy in patients with metastatic gastric or junctional adenocarcinoma (RAINFALL): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 20 , 420–435 (2019).
Rivera, F. et al. Perioperative trastuzumab, capecitabine and oxaliplatin in patients with HER2-positive resectable gastric or gastro-oesophageal junction adenocarcinoma: NEOHX phase II trial. Eur. J. Cancer 145 , 158–167 (2021).
Al-Batran, S.-E. et al. Final results and subgroup analysis of the PETRARCA randomized phase II AIO trial: Perioperative trastuzumab and pertuzumab in combination with FLOT versus FLOT alone for HER2 positive resectable esophagogastric adenocarcinoma [abstract 1421MO]. Ann. Oncol. 31 (Suppl. 4), S899 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02205047 (2022).
Bartley, A. N. et al. HER2 testing and clinical decision making in gastroesophageal adenocarcinoma: guideline from the College of American Pathologists, American Society for Clinical Pathology, and the American Society of Clinical Oncology. J. Clin. Oncol. 35 , 446–464 (2017).
Haffner, I. et al. HER2 expression, test deviations, and their impact on survival in metastatic gastric cancer: results from the prospective multicenter VARIANZ study. J. Clin. Oncol. 39 , 1468–1478 (2021).
Gomez-Martin, C. et al. Level of HER2 gene amplification predicts response and overall survival in HER2-positive advanced gastric cancer treated with trastuzumab. J. Clin. Oncol. 31 , 4445–4452 (2013).
Kaito, A. et al. HER2 heterogeneity is a poor prognosticator for HER2-positive gastric cancer. World J. Clin. Cases 7 , 1964–1977 (2019).
Kim, J. et al. Preexisting oncogenic events impact trastuzumab sensitivity in ERBB2-amplified gastroesophageal adenocarcinoma. J. Clin. Invest. 124 , 5145–5158 (2014).
Augustin, J. E., Soussan, P. & Bass, A. J. Targeting the complexity of ERBB2 biology in gastroesophageal carcinoma. Ann. Oncol. https://doi.org/10.1016/J.ANNONC.2022.08.001 (2022).
Shitara, K. et al. Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer. N. Engl. J. Med. 382 , 2419–2430 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04704934 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03602079 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03821233 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03255070 (2022).
Bang, Y. J. et al. First-in-human phase 1 study of margetuximab (MGAH22), an Fc-modified chimeric monoclonal antibody, in patients with HER2-positive advanced solid tumors. Ann. Oncol. 28 , 855–861 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04499924 (2022).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT05152147 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03615326 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04379596 (2022).
Catenacci, D. V. T. et al. Margetuximab plus pembrolizumab in patients with previously treated, HER2-positive gastro-oesophageal adenocarcinoma (CP-MGAH22-05): a single-arm, phase 1b-2 trial. Lancet Oncol. 21 , 1066–1076 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04082364 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04430738 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04276493 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03650348 (2021).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT05190445?term=NCT05190445&draw=2&rank=1 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04639219 (2022).
Yamaguchi, K. et al. Trastuzumab deruxtecan (T-DXd; DS-8201) in patients with HER2-low, advanced gastric or gastroesophageal junction (GEJ) adenocarcinoma: results of the exploratory cohorts in the phase II, multicenter, open-label DESTINY-Gastric01 study [abstract 1422MO]. Ann. Oncol. 31 (Suppl. 4), S899–S900 (2020).
Alexandrov, L. B. et al. Signatures of mutational processes in human cancer. Nature 500 , 415–421 (2013).
Kang, Y.-K. et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 390 , 2461–2471 (2017).
Fuchs, C. S. et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. 4 , e180013 (2018).
Bang, Y. J. et al. Phase III, randomised trial of avelumab versus physician’s choice of chemotherapy as third-line treatment of patients with advanced gastric or gastro-oesophageal junction cancer: primary analysis of JAVELIN Gastric 300. Ann. Oncol. 29 , 2052–2060 (2018).
Shitara, K. et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet 392 , 123–133 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04508140 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04879368?term=NCT04879368&draw=2&rank=1 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04752358 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04594811 (2022).
Shitara, K. et al. Efficacy and safety of pembrolizumab or pembrolizumab plus chemotherapy vs chemotherapy alone for patients with first-line, advanced gastric cancer: the KEYNOTE-062 phase 3 randomized clinical trial. JAMA Oncol. 6 , 1571–1580 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03675737 (2022).
Kang, Y. K. et al. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): a randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 23 , 234–247 (2022).
Xu, J. et al. Sintilimab plus chemotherapy (chemo) versus chemo as first-line treatment for advanced gastric or gastroesophageal junction (G/GEJ) adenocarcinoma (ORIENT-16): First results of a randomized, double-blind, phase III study [abstract LBA53]. Ann. Oncol. 32 (Suppl. 5), S1331 (2021).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03777657 (2022).
Xu, R. et al. RATIONALE 305: tislelizumab plus chemotherapy versus placebo plus chemotherapy as first-line therapy in patients with gastric or gastroesophageal junction adenocarcinoma [abstract P-26]. Ann. Oncol. 31 (Suppl. 3), S97–S98 (2020).
Moehler, M. et al. Phase III trial of avelumab maintenance after first-line induction chemotherapy versus continuation of chemotherapy in patients with gastric cancers: results From JAVELIN Gastric 100. J. Clin. Oncol. 39 , 966–977 (2021).
Marabelle, A. et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: results from the phase II KEYNOTE-158 study. J. Clin. Oncol. 38 , 1–10 (2020).
Chao, J. et al. Assessment of pembrolizumab therapy for the treatment of microsatellite instability-high gastric or gastroesophageal junction cancer among patients in the KEYNOTE-059, KEYNOTE-061, and KEYNOTE-062 Clinical Trials. JAMA Oncol. 7 , 895–902 (2021).
Janjigian, Y. et al. Nivolumab (NIVO) plus chemotherapy (Chemo) or ipilimumab (IPI) vs chemo as first-line (1L) treatment for advanced gastric cancer/gastroesophageal junction cancer/esophageal adenocarcinoma (GC/GEJC/EAC): CheckMate 649 study [abstract LBA7]. Ann. Oncol. 32 (Suppl. 5), S1329–S1330 (2021).
Kim, S. T. et al. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat. Med. 24 , 1449–1458 (2018).
Shitara, K. et al. The association of tissue tumor mutational burden (tTMB) using the Foundation Medicine genomic platform with efficacy of pembrolizumab versus paclitaxel in patients (pts) with gastric cancer (GC) from KEYNOTE-061 [abstract]. J. Clin. Oncol. 38 (Suppl. 15), 4537 (2020).
Khalid, S. et al. Association of TMB using the Foundation Medicine Companion Diagnostic (F1CDx) with efficacy of first-line pembrolizumab (pembro) or pembro plus chemotherapy (pembro + chemo) versus chemo in patients with gastric cancer (gc) from KEYNOTE-062 [abstract 1442P]. Ann. Oncol. 31 (Suppl. 4), S907–S908 (2020).
Janjigian, Y. et al. Co-occurring HER2 and PD-L1 expression in patients with HER2-positive trastuzumab-refractory gastric cancer (GC)/gastroesophageal junction adenocarcinoma (GEJA): biomarker analysis from the trastuzumab deruxtecan (T-DXd) DESTINY-Gastric03 trial [abstract SO-7]. Ann. Oncol. 33 (Suppl. 4), S358–S359 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04662710 (2022).
Palmer, A. C., Izar, B., Hwangbo, H. & Sorger, P. K. Predictable clinical benefits without evidence of synergy in trials of combination therapies with immune-checkpoint inhibitors. Clin. Cancer Res. 28 , 368–377 (2022).
Ji, J. et al. AK104 (PD-1/CTLA-4 bispecific) combined with chemotherapy as first-line therapy for advanced gastric (G) or gastroesophageal junction (GEJ) cancer: updated results from a phase Ib study [abstract]. J. Clin. Oncol. 39 (Suppl. 3), 232 (2021).
Kamada, T. et al. PD-1+ regulatory T cells amplified by PD-1 blockade promote hyperprogression of cancer. Proc. Natl Acad. Sci. USA 116 , 9999–10008 (2019).
Liu, Y. et al. Camrelizumab combined with FOLFOX as neoadjuvant therapy for resectable locally advanced gastric and gastroesophageal junction adenocarcinoma [abstract]. J. Clin. Oncol. 38 (Suppl. 15), 4536 (2020).
Li, H. et al. Phase II study of perioperative toripalimab in combination with FLOT in patients with locally advanced resectable gastric/gastroesophageal junction (GEJ) adenocarcinoma [abstract]. J. Clin. Oncol. 39 (Suppl. 15), 4050 (2021).
Alcindor, T. et al. Phase II trial of perioperative chemotherapy + avelumab in locally advanced gastroesophageal adenocarcinoma: Preliminary results [abstract]. J. Clin. Oncol. 39 (Suppl. 15), 4046 (2021).
Alsina, M. et al. MONEO: a phase II study of avelumab (Av) plus FLOT in the peri-operative treatment for patients (pts) with resectable gastric or gastroesophageal junction cancer (GC) [abstract]. J. Clin. Oncol. 39 (Suppl. 15), TPS4155 (2021).
Bang, Y. J. et al. KEYNOTE-585: Phase III study of perioperative chemotherapy with or without pembrolizumab for gastric cancer. Futur. Oncol. 15 , 943–952 (2019).
Article CAS Google Scholar
Janjigian, Y. Y. et al. MATTERHORN: efficacy and safety of neoadjuvant-adjuvant durvalumab and FLOT chemotherapy in resectable gastric and gastroesophageal junction cancer–a randomized, double-blind, placebo-controlled, phase 3 study [abstract]. J. Clin. Oncol. 39 (Suppl. 15), TPS4151 (2021).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04745988?term=NCT04745988&draw=2&rank=1 (2021).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03443856?term=NCT03443856&draw=2&rank=1 (2022).
Kelly, R. J. et al. Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer. N. Engl. J. Med. 384 , 1191–1203 (2021).
André, T. et al. Neoadjuvant nivolumab plus ipilimumab and adjuvant nivolumab in localized deficient mismatch repair/microsatellite instability-high gastric or esophagogastric junction adenocarcinoma: the GERCOR NEONIPIGA phase II study. J. Clin. Oncol. https://doi.org/10.1200/JCO.22.00686 (2022).
Hanahan, D. Hallmarks of cancer: new dimensions. Cancer Discov. 12 , 31–46 (2022).
Fuchs, C. S. et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 383 , 31–39 (2014).
Wilke, H. et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 15 , 1224–1235 (2014).
Li, J. et al. Randomized, double-blind, placebo-controlled phase III trial of apatinib in patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J. Clin. Oncol. 34 , 1448–1454 (2016).
Kang, Y.-K. et al. Randomized phase III ANGEL study of rivoceranib (apatinib)+best supportive care (BSC) vs placebo+BSC in patients with advanced/metastatic gastric cancer who failed ≥2 prior chemotherapy regimens [abstract LBA43]. Ann. Oncol. 30 (Suppl. 5), v877–v878 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT05029453?term=NCT05029453&draw=2&rank=1 (2021).
Al-Batran, S.-E. et al. Perioperative FLOT plus ramucirumab versus FLOT alone for resectable esophagogastric adenocarcinoma–updated results and subgroup analyses of the randomized phase II/III trial RAMSES/FLOT7 of the German AIO and Italian GOIM [abstract 1424MO]. Ann. Oncol. 31 (Suppl. 4), S901 (2020).
Van Cutsem, E. et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a biomarker evaluation from the AVAGAST randomized phase III trial. J. Clin. Oncol. 30 , 2119–2127 (2012).
Maron, S. B. et al. Targeted therapies for targeted populations: anti-EGFR treatment for EGFR-amplified gastroesophageal adenocarcinoma. Cancer Discov. 8 , 696–713 (2018).
Sahasrabudhe, R. et al. Germline mutations in PALB2, BRCA1, and RAD51C, which regulate DNA recombination repair, in patients with gastric cancer. Gastroenterology 152 , 983–986.e6 (2017).
Bang, Y. J. et al. Olaparib in combination with paclitaxel in patients with advanced gastric cancer who have progressed following first-line therapy (GOLD): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 18 , 1637–1651 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03427814 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02678182 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04209686?term=NCT04209686&draw=2&rank=1 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03008278 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03995017 (2022).
Lang, S. A. et al. Mammalian target of rapamycin is activated in human gastric cancer and serves as a target for therapy in an experimental model. Int. J. Cancer 120 , 1803–1810 (2007).
Lee, J. et al. Phase II trial of capecitabine and everolimus (RAD001) combination in refractory gastric cancer patients. Invest. New Drugs 31 , 1580–1586 (2013).
Ohtsu, A. et al. Everolimus for previously treated advanced gastric cancer: results of the randomized, double-blind, phase III GRANITE-1 study. J. Clin. Oncol. 31 , 3935–3943 (2013).
Kim, H. S., Kim, J. H. & Jang, H. J. Pathologic and prognostic impacts of FGFR2 amplification in gastric cancer: a meta-analysis and systemic review. J. Cancer 10 , 2560–2567 (2019).
Catenacci, D. V. T. et al. Phase I escalation and expansion study of bemarituzumab (FPA144) in patients with advanced solid tumors and FGFR2b-selected gastroesophageal adenocarcinoma. J. Clin. Oncol. 38 , 2418–2426 (2020).
Catenacci, D. V. T. et al. FIGHT: A randomized, double-blind, placebo-controlled, phase II study of bemarituzumab (bema) combined with modified FOLFOX6 in 1L FGFR2b + advanced gastric/gastroesophageal junction adenocarcinoma (GC) [abstract]. J. Clin. Oncol. 39 (Suppl. 15), 4010 (2021).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT05052801 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT05111626 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04604132 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02052778 (2022).
Van Cutsem, E. et al. A randomized, open-label study of the efficacy and safety of AZD4547 monotherapy versus paclitaxel for the treatment of advanced gastric adenocarcinoma with FGFR2 polysomy or gene amplification. Ann. Oncol. 28 , 1316–1324 (2017).
Hashimoto, I. & Oshima, T. Claudins and gastric cancer: an overview. Cancers 14 , 290 (2022).
Sahin, U. et al. FAST: a randomised phase II study of zolbetuximab (IMAB362) plus EOX versus EOX alone for first-line treatment of advanced CLDN18.2-positive gastric and gastro-oesophageal adenocarcinoma. Ann. Oncol. 32 , 609–619 (2021).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03653507 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03504397 (2022).
Qi, C. et al. Claudin18.2-specific CAR T cells in gastrointestinal cancers: phase 1 trial interim results. Nat. Med. 28 , 1189–1198 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03874897 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04400383?term=NCT04400383&draw=2&rank=1 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04404595 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04467853?term=NCT04467853&draw=2&rank=1 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04260191 (2022).
Alsina, M., Diez, M. & Tabernero, J. Emerging biological drugs for the treatment of gastroesophageal adenocarcinoma. Expert. Opin. Emerg. Drugs 26 , 385–400 (2021).
Lee, J. et al. Tumor genomic profiling guides patients with metastatic gastric cancer to targeted treatment: the Viktory Umbrella Trial. Cancer Discov. 9 , 1388–1405 (2019).
Yuki, S. et al. The nationwide cancer genome screening project in Japan SCRUM-Japan GI-SCREEN: efficient identification of cancer genome alterations in advanced gastric cancer (GC) [abstract]. J. Clin. Oncol. 36 (Suppl. 15), 4050 (2018).
Nakamura, Y. et al. Clinical utility of circulating tumor DNA sequencing in advanced gastrointestinal cancer: SCRUM-Japan GI-SCREEN and GOZILA studies. Nat. Med. 26 , 1859–1864 (2020).
Wainberg, Z. A. et al. Randomized double-blind placebo-controlled phase 2 study of bemarituzumab combined with modified FOLFOX6 (mFOLFOX6) in first-line (1L) treatment of advanced gastric/gastroesophageal junction adenocarcinoma (FIGHT) [abstract]. J. Clin. Oncol. 39 (Suppl. 3), 160 (2021).
Dykewicz, C. A. Summary of the guidelines for preventing opportunistic infections among hematopoietic stem cell transplant recipients. Clin. Infect. Dis. 33 , 139–144 (2001).
Download references
Author information
Authors and affiliations.
Gastrointestinal and Endocrinology Group, Vall d’Hebron Institute of Oncology (VHIO), Barcelona, Spain
Maria Alsina, Marc Diez & Josep Tabernero
Medical Oncology Department, Hospital Universitario de Navarra (HUN), Pamplona, Spain
Maria Alsina & Virginia Arrazubi
Oncobiona Group, Instituto de Investigación Sanitaria de Navarra (IdiSNA), Pamplona, Spain
Medical Oncology Department, Hospital Universitari Vall d’Hebron (HUVH), Barcelona, Spain
Marc Diez & Josep Tabernero
You can also search for this author in PubMed Google Scholar
Contributions
M.A., V.A. and M.D. researched data for the article. M.A. and V.A. contributed substantially to discussion of the content. M.A., V.A. and M.D. wrote the article. M.A., V.A. and J.T. reviewed and/or edited the manuscript before submission.
Corresponding author
Correspondence to Josep Tabernero .
Ethics declarations
Competing interests.
M.A. declares consulting/advisory roles with BMS, Lilly, MSD and Servier; and honoraria for speaking from Amgen, BMS, Lilly, MSD and Servier. V.A. declares consulting/advisory roles with BMS and MSD; and honoraria for speaking from BMS and Lilly. M.D. reports a financial interest in the form of a scientific consultancy role for Lilly and travel expenses partially covered by Lilly. J.T. reports a personal financial interest in the form of a scientific consultancy role for Array Biopharma, AstraZeneca, Avvinity, Bayer, Boehringer Ingelheim, Chugai, Daiichi Sankyo, F. Hoffmann-La Roche Ltd, Genentech Inc, HalioDX SAS, Hutchison MediPharma International, Ikena Oncology, Inspirna Inc, IQVIA, Lilly, Menarini, Merck Serono, Merus, MSD, Mirati, Neophore, Novartis, Ona Therapeutics, Orion Biotechnology, Peptomyc, Pfizer, Pierre Fabre, Samsung Bioepis, Sanofi, Seattle Genetics, Scandion Oncology, Servier, Sotio Biotech, Taiho, Tessa Therapeutics and TheraMyc; and also educational collaborations with Imedex, Medscape Education, MJH Life Sciences, PeerView Institute for Medical Education and Physicians Education Resource (PER); and declares institutional financial interests in the form of financial support for clinical trials or contracted research for Amgen Inc, Array Biopharma Inc, AstraZeneca Pharmaceuticals LP, BeiGene, Boehringer Ingelheim, BMS, Celgene, Debiopharm International SA, F. Hoffmann-La Roche Ltd, Genentech Inc, HalioDX SAS, Hutchison MediPharma International, Janssen-Cilag SA, MedImmune, Menarini, Merck Health KGAA, Merck Sharp & Dohme, Merus NV, Mirati, Novartis Farmacéutica SA, Pfizer, Pharma Mar, Sanofi Aventis Recherche & Développement, Servier, Taiho Pharma USA Inc, Spanish Association Against Cancer Scientific Foundation and Cancer Research UK.
Peer review
Peer review information.
Nature Reviews Gastroenterology & Hepatology thanks Andres Cervantes, Yukinori Kurokawa and the other, anonymous, reviewer for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Cite this article.
Alsina, M., Arrazubi, V., Diez, M. et al. Current developments in gastric cancer: from molecular profiling to treatment strategy. Nat Rev Gastroenterol Hepatol 20 , 155–170 (2023). https://doi.org/10.1038/s41575-022-00703-w
Download citation
Accepted : 11 October 2022
Published : 07 November 2022
Issue Date : March 2023
DOI : https://doi.org/10.1038/s41575-022-00703-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Mbd3 promotes epithelial-mesenchymal transition in gastric cancer cells by upregulating actg1 via the pi3k/akt pathway.
- Huizhi Wang
Biological Procedures Online (2024)
LncRNA WFDC21P interacts with SEC63 to promote gastric cancer malignant behaviors by regulating calcium homeostasis signaling pathway
- Jinyao Dong
- Yongqiang Lv
- Yanyan Zhang
Cancer Cell International (2024)
GGT5 facilitates migration and invasion through the induction of epithelial–mesenchymal transformation in gastric cancer
BMC Medical Genomics (2024)
Characterization of alternative splicing events and prognostic signatures in gastric cancer
- Yupeng Zhao
Effects of perioperative low-dose naloxone on the immune system in patients undergoing laparoscopic-assisted total gastrectomy: a randomized controlled trial
- Xiangzhen Min
- Huaixin Xing
BMC Anesthesiology (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List

Surgical Management of Gastric Cancer: A Systematic Review
Lucian mocan.
1 Department of Surgery, Iuliu Hatieganu University of Medicine and Pharmacy, RO-400012 Cluj-Napoca, Romania; [email protected] or moc.oohay@naiculnacom ; Tel.: +40-745-362-345
2 Regional Institute of Gastroenterology and Hepatology, 19-21 Croitorilor Street, RO-400162 Cluj-Napoca, Romania
Associated Data
Not applicable.
Gastric cancer is the fifth most common cancer worldwide, and it is responsible for 7.7% of all cancer deaths. Despite advances in the field of oncology, where radiotherapy, neo and adjuvant chemotherapy may improve the outcome, the only treatment with curative intent is represented by surgery as part of a multimodal therapy. Two concepts may be adopted in appropriate cases, neoadjuvant treatment before gastrectomy (G) or primary surgical resection followed by chemotherapy. Such an approach, combined with early detection and better screening, has led to a decrease in the overall incidence of gastric cancer. Unfortunately, malignant tumors of the stomach are often diagnosed in locally advanced or metastatic stages when the median overall survival remains poor. Surgical care in these cases must be provided by a multidisciplinary team in a high-volume center. Important surgical aspects such as optimum resection margins, surgical technique, and number of harvested lymph nodes are important factors for patient outcomes. The standardization of surgical treatment of gastric cancer in accordance with the patient’s profile is of decisive importance for a better outcome. This review aims to summarize the current standards in the surgical treatment of gastric cancer.
1. Introduction
Gastric cancer is the fifth most common cancer worldwide, and it is responsible for 7.7% of all cancer deaths. Although surgical treatment for gastric cancer has been considerably improved during recent decades, the mortality rate from gastric cancer is still high [ 1 ]. Statistical data show that the 5-year survival rate for patients treated with curative intent (gastric resection and lymphadenectomy) is 70% for stage I resected gastric cancer and less than 30% for stage IIB disease and beyond [ 2 ]. Most gastric tumors are adenocarcinomas [ 3 ]. Despite advances in the field of oncology, where radiotherapy, neo and adjuvant chemotherapy may improve the outcome, the only treatment with curative intent is represented by surgery as part of a multimodal therapy [ 4 ]. Two concepts may be adopted in appropriate cases, neoadjuvant treatment before gastrectomy or primary surgical resection followed by chemotherapy [ 5 ].
Genetic alterations responsible for the development and progression of gastric cancer such as cell adhesion, signal transduction, DNA methylation, and glycosylation changes may lead to early detection of gastric cancers using these biomarkers [ 6 ].
For patients with hereditary diffuse gastric cancer (HDGC), who carry a lifetime gastric cancer risk of approximately 70% in men and 56% in women, a prophylactic total gastrectomy at the age of 20 years is the procedure of choice [ 7 ]. Recently, there have been numerous sources of evidence establishing the importance of combining systemic chemotherapy with surgery in advanced gastric cancer. Given the latest results, there has been a shift in the paradigm of gastric cancer treatment with the increasing use of preoperative and postoperative chemotherapy [ 8 ].
Unfortunately, malignant tumors of the stomach are often diagnosed in locally advanced or metastatic stages when the median overall survival remains poor [ 9 ]. Surgical care in these cases must be provided by a multidisciplinary team in a high-volume center [ 10 ]. Important surgical aspects such as optimum resection margins, surgical technique, number of harvested lymph nodes are important factors for patient outcomes. The standardization of surgical treatment of gastric cancer in accordance with the patient’s profile is of decisive importance for a better outcome ( Figure 1 ). This review aims to summarize the current standards in the surgical treatment of gastric cancer.

Treatment strategies of gastric cancer according to TNM stage. Stage 0: TisN0M0; stage IA: T1N0M0; stage IB: T2N0M0; stage II: T1N2M0/T2N1M0/T3N0M0; stage IIIA: T3N1M0/T4N0M0; stage IIIB: T3N2M0; stage IV: T3N1-3M0/T1-3N3M0/T1-4N0-3M1 (Tis—the mucosa; T1—submucosa;T2—muscle layer; T3—subserosa; T4—serosa/adjacent structures/N0—(0+)LN; N1—(1–2+)LN; N2—(3–6+)LN; N3—(>7+)LN/M0—no metastasis; M1—distant metastasis or carcinomatosis); LN—lymph nodes; ST—subtotal; T—total; ChT—chemotherapy; ChRxT—chemo-radiotherapy; preop—preoperative; postop—postoperative.
2. Results and Discussion
2.1. extent of gastric resection: total gastrectomy (tg), subtotal gastrectomy (sg), and proximal gastrectomy (pg).
The extent of surgical resection required to achieve surgical margins free of malignant cells, R0, depends on the size, location, and histological type of the tumor. The optimal length for the proximal margin is often suggested to be at least 3 to 5 cm depending on the tumor histology [ 11 ]. However, recent studies suggest that resection margins of 1 cm may be comparable in terms of survival and oncological outcome [ 12 ].
Since the standard approach for gastric cancer with any localization is total gastrectomy (TG), several studies have shown that the outcomes of patients with proximal tumors who underwent TG or proximal gastrectomy (PG) were similar in terms of the overall survival interval and disease-free interval [ 13 ]. Following these studies, it is accepted today that both procedures could be accomplished safely. Some authors suggest that distal gastrectomy can be safely performed for patients with distal lesions and TG/PG may be performed for proximal lesions [ 14 ]. The benefit of PG for the surgical treatment of proximal cancers was assessed by Harrison et al. [ 15 ], and the researchers showed that patients with proximal tumors who underwent PG resection had similar overall survival and disease-free intervals compared with patients who underwent TG resection. The only concern related to PG was represented by the increased number of patients who experienced late complications, such as esophageal reflux [ 16 ]. Several trials [ 17 , 18 , 19 , 20 , 21 ] addressed the issues of postoperative mortality, morbidity, and long-term outcome of TG versus subtotal gastrectomy (SG) for distal tumors. The wound infection, anastomotic fistula, and mortality rates were higher in the TG group compared with the SG group. However, there was no difference in the postoperative mortality rate or 5-year survival rate between the two groups (TG vs. SG). For patients who underwent radical SG, studies have shown an improved quality of life compared with the TG group [ 22 ].
The controversy continues in tumors of the cardia when radical resection requires supradiaphragmatic anastomosis [ 23 ]. There are three therapeutic options in such cases: some surgeons prefer PG, whereas others adopt either TG or esophagectomy with proximal cervical anastomosis as the ideal therapeutic option. Ito et al. [ 24 ] showed no difference between total esophagectomy, thoracic esophagogastrectomy, and abdominal esophagogastrectomy in terms of 5-year survival rates. Postoperative complications were significantly higher in patients with esophagectomy (33% vs. 11%). In these cases, for the optimal (R0) resection, >4 cm (distal) gastric margin and >6 cm esophageal margin must be considered. The actual recommendations are to avoid the transabdominal approach only for the extent of esophageal resection in tumors involving the distal esophagus and cardia. In such cases, an individualized operative approach is recommended for an optimal R0 resection [ 25 ]. For patients who have extensive linitis plastica, TG is more frequently performed [ 26 ]. Recent reports have indicated that, if R0 can be safely achieved, pylorus-preserving resection for gastric cancer should be considered because no difference in survival was reported compared with more extensive procedures [ 27 , 28 ].
2.2. Reconstruction Following Resection
The technique of anastomosis that began in 1881, when Theodor Billroth performed the first gastrectomy, has been extensively explored in numerous studies since then [ 29 ]. Although Billroth I was the method of choice for a long time, it is currently accepted that the bile reflux gastritis is best minimized by Roux-en-Y reconstruction [ 30 ]. However, until the 21st century, the general preference for TG was to use jejunum loop reconstruction; during the last two decades, Roux-en-Y reconstruction became the standard procedure worldwide [ 31 ]. The Roux-en-Y reconstruction following TG is also a preferred method of reconstruction after pancreatic and biliary resections and liver and pancreatic cysts, as well as in bariatric surgery [ 32 ].
The reconstruction using jejunal pouches has historical value, but such pouches are no longer employed because they show limited benefit. Small-bowel interposition was preferred in the past by some surgeons; today, it has less acceptance and is rarely used [ 33 ].
2.3. Extending Resection to Adjacent Organs
Extended resection (D2 resection with splenectomy and distal pancreatectomy) for advanced gastric cancer, initially performed by Japanese surgeons, has been a subject of debate for decades [ 34 ]. Although early reports showed improved survival [ 35 ], large prospective randomized control trials failed to report a real survival benefit. In patients with splenectomy and distal pancreatectomy, higher morbidity, higher mortality, and longer hospital stays were observed [ 36 ]. In a study published by Otsuji et al. [ 37 ], out of 128 patients who underwent TG for gastric adenocarcinoma of the middle or proximal stomach, 35.9% underwent pancreatosplenectomy (PS), 44.6% underwent splenectomy (S), and underwent 19.5% gastrectomy alone. The morbidity and mortality were higher in patients with pancreatosplenectomy, mainly due to pancreatic fistula occurrence. Five-year survival rates of 40.7% (PS), 55.9% (S), and 54.2% (G) were reported. Importantly, in multivariate analysis, PS and S alone were found to not be independent factors for survival, strongly suggesting that PS increases morbidity rates without improving survival.
A series of 353 patients who underwent extended resection of the adjacent organs (removal of transverse colon in 45%, pancreas and spleen in 42.5%, left hepatic lobe in 28.5%, and head of the pancreas in 10.5%) was published by Shchepotin et al. [ 38 ]. TG was performed in 32.9% of patients and SG in 67.1%. The lymphadenectomy was standardly performed in all patients (lymph nodes around stomach, celiac axis, hepatic artery, and proximal splenic artery). The presented data displayed a 5-year survival rate of 25% (37% for N− and 15% for N+).
Data arising from two prospective randomized control trials that do not favor gastrectomy with additional organ resection have been published [ 39 , 40 ]. Bonenkamp et al. [ 41 ] reported the results of extended gastrectomy on 996 patients randomized to a D1 or D2 lymph node dissection. A significant increase in postoperative complications, reoperation rates, and hospital stays was seen in patients requiring a D2 lymphadenectomy [ 42 ]. D2 with splenectomy and/or pancreatectomy was significantly responsible for this poor outcome [ 43 ]. In another paper, Kasakura et al. [ 44 ] showed that removal of an additional organ was not a factor for survival in stages II, III, and IV. We can conclude that extended resection (where R0 is feasible) of the adjacent organs can be performed by highly experienced surgeons in patients with T4 tumors.
2.4. Extent of Lymphadenectomy
The extent of lymphadenectomy performed along with gastrectomy has been a debated subject for decades [ 45 , 46 , 47 , 48 ]. The concept was first described by the Japanese Research Society for Gastric Cancer (JRSGC) in 1973 [ 49 ]. Comparisons between limited D1 (perigastric lymph nodes), extended D2 (perigastric and celiac axis lymph node stations), and D3 (perigastric, celiac axis, and para-aortic lymph node stations) lymphadenectomies have been analyzed for decades in prospective randomized trials.
Some authors suggest that the oncological benefit of extended nodal resection does not overcome the drawbacks of postoperative morbidity and mortality. Most Western surgeons consider that extended nodal dissection has no benefit for overall survival and malignant lymph nodes are prognostic indicators rather than factors of survival. Other surgeons (e.g., Japanese surgeons) think that the optimal therapy associated with better loco-regional control is radical gastrectomy with extensive lymphadenectomy [ 50 ]. These facts have also been confirmed by several experienced surgeons who performed complete D2 lymphadenectomy and showed that complications are no higher for D2 in surgeries performed by experienced surgeons; however, the 5-year survival rate because of prevention of loco-regional recurrences is significantly higher in those patients [ 51 ].
A highly cited Dutch trial was conducted by the Dutch Gastric Cancer Group from August 1989 to July 1993 [ 52 ]. The researchers randomized D1 and D2 dissection into two groups (711 patients in total). The D1 lymphadenectomy addressed perigastric lymph nodes only, and extended D2 lymphadenectomy incorporated additional clearance of celiac axis lymph nodes. Distal pancreatectomy with splenectomy was routinely performed for D2 completion. The published results of the trial showed a higher postoperative morbidity (43% vs. 4%, p < 0.001) and mortality (10% vs. 4%, p < 0.004) in the D2 lymphadenectomy group compared with the other group. Importantly, no difference in 5-year survival between the two groups (34% in D1 vs. 33% in D2) was observed. The unanimous conclusion following this trial was that routinely performed D2 lymphadenectomy in gastric cancer patients has no benefits for long-term survival.
The same Dutch Gastric Cancer Trial (DGCT) group recently reported data from a 15-year follow up after the above randomized nationwide Dutch D1/D2 trial and showed that disease-specific survival was significantly higher in patients receiving D2 versus D1 lymphadenectomy, but there was no improvement in overall survival [ 53 ].
The MRC trial, led by Alfred Cuschieri et al. [ 54 ], was a large, multicenter trial (32 surgeons) including 400 patients, who were divided into two groups. In one group, 200 patients underwent D1 dissection (lymphadenectomy within 3.0 cm of the tumor survival); in the other group, the remaining 200 patients had D2 dissection (lymphadenectomy of the omental bursa, hepatoduodenal nodes, and retroduodenal nodes for distal cancers and the splenic artery/splenic pedicle nodes for proximal cancers). In this trial, the postoperative morbidity and mortality were significantly higher in the D2 group (D2 vs. D1: 46% vs. 28%, p < 0.001; 13% vs. 6.5%; p = 0.04) The obtained results were comparable in terms of 5-year survival rates (35% for D1 resection and 33% for D2), gastric cancer-specific survival (hazard ratio (HR) = 1.05, 95% confidence interval (CI): 0.79–1.39), and recurrence-free survival (HR = 1.03, 95% CI: 0.82–1.29). Based on the findings of the trial, the authors suggested that classical Japanese D2 resection offered no survival advantage over D1 resection.
Following the criticism of the Dutch trial because of the high complication rate, an Italian phase II study [ 55 ] was proposed to clarify the importance of D2 dissection. To avoid potential bias, only surgeons with extensive experience in gastric cancer surgery were allowed to participate. In 191 patients with D2 lymphadenectomy (with spleen preservation), the authors showed almost similar morbidity rates following D1 and D2 lymph node dissection (12.0% vs. 17.9%, p = 0.178); there were also comparable results in terms of the 30-day postoperative mortality rates (D1 vs. D2: 3.0% vs. 2.2%, p = 0.72).
To assess the importance of an extended D2 (para-aortic lymph nodes) resection following gastric resection for cancer, a randomized trial was conducted by East Asia Surgical Oncology [ 56 ], in which 269 patients were divided into two groups. There were 135 patients in the D2 resection group and 134 in the D2+ para-aortic lymphadenectomy group. The authors reported comparable 5-year survival intervals between the two groups (52.6% for D2 vs. 55.0% for D2+, χ2 = 0.064; p = 0.80). The presented data failed to impose prophylactic para-aortic lymphadenectomy as a standard technique in gastric cancer treatment.
A significantly better disease-specific survival was observed in D2 compared with D1 lymphadenectomy in a Cochrane systematic review (Hazard Ratio 0.81, 95% CI: 0.71–0.92), although the rate of mortality was higher in the D2 group (Risk Ratio 2.02, 95% CI: 1.34–3.04). No statistically significant difference was observed in the disease-free interval between the D1 and D2 groups [ 57 ]. The Japan Clinical Oncology Group (JCOG) trial 9501 compared D2 and D3 lymphadenectomy [ 58 ]. The D3 lymphadenectomy (additional dissection of para-aortic lymph nodes) group had a higher morbidity rate, but the overall 5-year survival and local recurrence were the same between the two groups. Data analysis from the Surveillance, Epidemiology, and End-Results (SEER) database has shown that survival benefits occurred in patients with gastric resections who had >15 lymph nodes excised during gastrectomy [ 59 ]. Although D2 lymphadenectomy is not mandatory for gastric cancer treatment, it is strongly recommended. The current The National Comprehensive Cancer Network NCCN guidelines sustain that lymphadenectomy should remove at least 15 nodes to optimize oncologic outcomes in gastric cancer [ 60 ].
Anastomotic leakage, pancreatic leakage, and higher reoperation rates were associated with D2 dissection in the MRC [ 55 ] and Dutch trials [ 41 ], mainly because of inadequate surgical training in splenectomy and pancreatectomy rather than D2 itself. An Italian trial showed for the first time that D2 dissection without splenectomy and distal pancreatectomy has the same mortality and morbidity as the same surgery does for D1 dissection [ 61 ]. Routinely performed splenectomy or distal pancreatectomy may be considered efficient only when the primary tumor or metastatic lymph nodes directly invade the pancreas and spleen [ 62 ].
2.5. Influence of Positive Resection Margins and Re-Resection
Recent studies have shown that the incidence of positive margins following extended resection for advanced gastric cancer is around 24% [ 63 ]. This rate includes reports from pathology examinations (R1) and macroscopic validation of malignant tissue on resection margins (R2) [ 64 ].
A retrospective study was conducted by Cho et al. [ 65 ] over a 15-year period. Of the 2740 patients included, 49 (1.8%) had positive margins (29 proximal and 20 distal), and multivariate analysis identified extragastric extension and total gastrectomy as independent risk factors for positive resection margins ( p = 0.015 and p = 0.014, respectively). Long-term survival was also significantly lower in patients with positive margins than in those with negative margins ( p = 0.0028 and p = 0.025, respectively).
Multiple prospective randomized trials regarding the influence of the R1 margin on gastric cancer survival have been conducted in Asian and Western populations, but the results have been controversial [ 66 , 67 , 68 ]. Several authors found that positive margins are an independent risk factor for survival following gastric resection for cancer [ 69 ]. A multivariate regression analysis performed by Bickenbach [ 70 ] showed that R1 margins were associated with poor survival, but this association was only observed in patients with fewer than three positive lymph nodes or T1–2 disease. Schoenfeld et al. [ 68 ] showed that R1 margins were associated with a lower disease-free interval, but overall survival rates were comparable to those for R0. Regarding the opportunity for a re-resection following R1, Raziee et al. [ 69 ] obtained controversial results via a systematic review, but they agreed that re-resection should be performed to eliminate the R1 margin whenever feasible [ 71 ].
2.6. The Importance of High-Volume Centers
It has been stated in previous studies that high-volume departments are significantly associated with better survival, lower mortality and morbidity, and lower reoperation rates following resection for gastric cancer [ 72 , 73 , 74 , 75 ]. However, these studies have been criticized for a lack of statistical power because most have been retrospective reviews with few patient data [ 73 , 75 ]. Moreover, they have neglected major variables, such as recurrence rates, adjuvant therapy and long-term follow-up. An intergroup trial [ 76 ] that assessed the implementation of D2 lymphadenectomy in US hospitals showed that in 556 patients with adjuvant chemoradiotherapy and gastric resection, 54% had incomplete lymphadenectomy (less than D1), 37% had D1, and only 9% had D2. The underlying factor was the surgeon’s experience. Other studies have confirmed this point, showing that fewer than 33% of patients with curative gastrectomies had 15 or more lymph nodes removed/examined [ 77 , 78 ]. It has been proven using computer-based models that poor dissections of lymph nodes because of inadequate surgical techniques lead to poor survival in these patients [ 79 , 80 ].
An extensive meta-analysis analyzed 28 papers describing the relationship between hospital volume and surgeons’ experience; the 5-year survival showed that high-volume hospitals have fewer complications and better outcomes following gastric resection for cancer [ 81 ]. The number of procedures (gastric resection with D2) and the surgeons’ level of training and supraspecialization are key factors related to low postoperative complications, low gastrectomy-related mortality, and better five-year survival.
Studies suggest that procedure-related mortality is significantly higher in US hospitals, ranging from 5% to 13% [ 82 ]. Large statistical data examining more than 600 hospitals in the US over a 5-year period showed that the average perioperative mortality rate was 7.2%. Therefore, based on the above results and from our personal experience, we state that D2 lymphadenectomy and gastric surgery for cancer should be performed in tertiary surgical centers where surgeons are routinely performing this type of operation and have very low operative morbidity and mortality rates.
2.7. Laparoscopic Gastrectomy (LG)
Laparoscopic resection of gastric cancer is routinely performed worldwide [ 83 ] and has become a popular approach for treating gastric cancer in Asian countries (representing 25% of all gastric resections for cancer in Japan and South Korea) [ 84 , 85 ]. The surgical techniques and postoperative outcome have been well established in two prospective trials (KLASS 01 and JCOG 0703) for early gastric cancer [ 86 , 87 ].
A Japanese study (LOC-1) included 3630 patients with early gastric cancer treated with laparoscopic gastrectomy (LG) or open gastrectomy (OG) between 2006 and 2012. [ 88 ]. There was no significant difference in the 5-year overall survival (97.1% for LG vs. 96.3% for OG) or local recurrence rate (2.3% vs. 2.4%).
In 2006–2010, a trial developed in South Korea (KLASS-1), which involved 1400 patients with invasive distal gastric cancer limited to the submucosa, analyzed the feasibility of laparoscopic distal gastrectomy [ 88 ]. The LG group had a lower morbidity rate (13% vs. 20%) and lower wound infections (3.6% vs. 7%) compared with the OG group. Major intra-abdominal complications and perioperative mortality rates were similar between the two groups. The overall 5-year survival rate was comparable between the two groups (LG: 94.2%; OG: 93.3%), as were the cancer-specific survival rates (LG: 97.1%; OG: 97.2 percent) [ 89 ].
LG is a complicated procedure that requires training and experience [ 90 , 91 ]; it also necessitates support from the staff and hospital. The technical pitfalls make LG controversial for the resection of locally advanced tumors, mainly because of concerns regarding the R0 acquisition and adequate D2 dissection [ 92 ]. However, patients with LGs report a better quality of life in the early postoperative period. Ultimately, patients with invasive gastric cancer that invades no more deeply than the submucosa, regardless of lymph node metastasis (T1, any N, M0), and who are free of significant cardiopulmonary diseases, obesity, and previous upper abdominal surgery, are most suitable for LG.
3. Conclusions
The standardization of surgical resection in accordance with tumor stage is of decisive importance for a better outcome. As gastrectomy and adequate lymph node resection may be challenging, the treatment must be provided by a multidisciplinary team in a high-volume center. Since neo and adjuvant chemotherapy improve the outcome, multimodal therapy is the treatment of choice in stage IB and above. If R0 is technically feasible, distal gastrectomy can be safely performed for patients with distal lesions while TG/PG may be performed for proximal lesions. Splenectomy or distal pancreatectomy should not be performed as part of D2 lymphadenectomy and may be considered only when the primary tumor or metastatic lymph nodes directly invade the pancreas and spleen. As surgical centers with higher volume have very low operative morbidity and mortality rates, patients proposed for D2 lymphadenectomy and gastric resection for cancer should be referred to these hospitals. Surgeons should perform re-resection to eliminate the R1 margin whenever this is feasible. Laparoscopic gastrectomy may be performed by experienced surgeons with no compromise in surgical principles.
The authors wish to acknowledge financial support Romanian National Authority for Scientific Research and Innovation, CNCS-UEFISCDI, project numbers, PN-III-P2-2.1-PED-2019-0844, PN-III-P2-2.1-PED-2019-0997, PN-III-P2-2.1-PED-2019-3373.
Institutional Review Board Statement
Informed consent statement, data availability statement, conflicts of interest.
The author declares no conflict of interest.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
- Open access
- Published: 27 May 2023
Gastric cancer treatment: recent progress and future perspectives
- Wen-Long Guan 1 , 2 na1 ,
- Ye He 1 , 2 na1 &
- Rui-Hua Xu 1 , 2
Journal of Hematology & Oncology volume 16 , Article number: 57 ( 2023 ) Cite this article
27k Accesses
78 Citations
11 Altmetric
Metrics details
Gastric cancer (GC) is one of the most common malignancies worldwide. Most patients are diagnosed at advanced stages due to the subtle symptoms of earlier disease and the low rate of regular screening. Systemic therapies for GC, including chemotherapy, targeted therapy and immunotherapy, have evolved significantly in the past few years. For resectable GC, perioperative chemotherapy has become the standard treatment. Ongoing investigations are exploring the potential benefits of targeted therapy or immunotherapy in the perioperative or adjuvant setting. For metastatic disease, there have been notable advancements in immunotherapy and biomarker-directed therapies recently. Classification based on molecular biomarkers, such as programmed cell death ligand 1 (PD-L1), microsatellite instability (MSI), and human epidermal growth factor receptor 2 (HER2), provides an opportunity to differentiate patients who may benefit from immunotherapy or targeted therapy. Molecular diagnostic techniques have facilitated the characterization of GC genetic profiles and the identification of new potential molecular targets. This review systematically summarizes the main research progress in systemic treatment for GC, discusses current individualized strategies and presents future perspectives.
Gastric cancer (GC) is the fifth most common malignant tumor and the fourth leading cause of cancer-associated death worldwide [ 1 , 2 ]. The incidence varies geographically across the globe, with the highest incidence in Eastern Asia (Japan and Mongolia) and Eastern Europe, whereas incidence rates in Northern Europe and Northern America are generally low, comparable to African regions [ 2 ]. Notably, the incidence of gastric cancer among young adults (aged < 50 years) in recent years has been progressively rising in both low-risk and high-risk countries. Aside from Helicobacter Pylori infection, the occurrence of GC has been linked to genetic risk factors as well as lifestyle factors, such as alcohol consumption and smoking [ 3 , 4 , 5 , 6 ].
Despite the high incidence of GC, most patients are unfortunately diagnosed at advanced stages with dismal prognoses due to the lack of distinguishing clinical indications [ 7 , 8 ]. Systemic chemotherapy is the mainstay treatment for metastatic GC (mGC), with a median overall survival (OS) of ~ 12 months for patients treated with conventional chemotherapy [ 9 ]. Intratumoral and intertumoral heterogeneity are the prominent features of GC that partly contribute to its poor prognosis. However, histological classifications alone are insufficient to effectively stratify patients for individualized treatment and improve patients’ clinical outcomes [ 10 ]. Therefore, cutting-edge diagnostic techniques and drugs are of fundamental importance for better characterizing molecular profiles and identifying potential novel therapeutic targets for GC patients [ 11 , 12 , 13 ].
Trastuzumab, a monoclonal antibody targeting Human Epidermal Receptor 2 (HER2), was the first approved targeted therapy for GC. However, after the ToGA study, progress in the development of treatments for gastric cancer stalled for nearly a decade [ 14 ]. Emerging advances in immunotherapy, particularly in anti-HER2 therapy, and various biomarker-directed therapies in GC have recently broken this trend. For example, anti-programmed cell death 1 (PD-1) antibodies have demonstrated impressive efficacy and prolonged survival in untreated MSI-H/dMMR mGC patients [ 15 ]. Substantial breakthroughs in the treatment of gastric cancer have been achieved with novel anti-HER2 therapeutic agents, such as T-DXd and disitamab vedotin (RC48) [ 16 ]. In addition, in light of the success of immunotherapy and targeted therapy as first-line treatments for advanced gastric cancer, ongoing research is investigating their potential to advance the treatment of patients with locally advanced stage GC.
The treatment landscape of gastric cancer has evolved significantly in the past few years, with the emergence of new immunotherapy and targeted therapies for patients at various stages of the disease (Fig. 1 ). In this review, we systematically summarize the pivotal clinical trials in GC treatment and provide an update on the management of localized and metastatic gastric cancer. We also discuss the developments in immunotherapy and targeted therapy and highlight current individualized treatments and future perspectives.
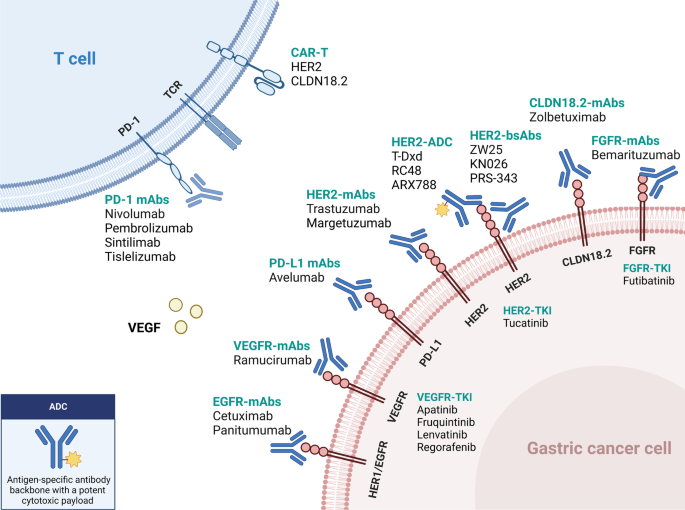
Updated immunotherapy and targeted therapy for gastric cancer. This algorithm provides guidance for selecting currently available immunotherapy and targeted therapy based on different biomarkers
Management for localized GC
Radical surgery is the primary treatment for resectable gastric cancer. Several therapeutic approaches have been established to lower the risk of recurrence and improve long-term survival, including perioperative chemotherapy, adjuvant chemotherapy, and adjuvant chemoradiotherapy (Table 1 ). They are listed as the recommended treatments for resectable localized GC in current guidelines[ 5 , 17 , 18 ]. Further, the addition of targeted therapy and/or immune checkpoint inhibitors (ICIs) is currently being studied in the neoadjuvant/adjuvant setting.
- Perioperative chemotherapy
Perioperative chemotherapy has become the standard treatment for resectable localized GC. Several clinical trials have demonstrated that perioperative chemotherapy could improve the prognosis of patients with resectable GC compared to surgery alone.
The MAGIC trial marked a significant advancement in the field of perioperative chemotherapy for resectable GC. In this phase 3 study, 503 patients were enrolled with resectable gastric, gastroesophageal junction (GEJ), or lower esophageal adenocarcinoma. Patients in the experimental group received three preoperative and three postoperative cycles of epirubicin, cisplatin, and fluorouracil (ECF) [ 19 ]. The results showed that the perioperative ECF regimen could decrease tumor stage and significantly improve progression-free survival (PFS, HR 0.66; 95% CI 0.53–0.81, P < 0.001) and overall survival (OS, HR 0.75; 95% CI 0.60–0.93, P = 0.009). Another phase III trial conducted in 28 French centers compared radical surgery with or without perioperative cisplatin and fluorouracil (CF) chemotherapy and showed that perioperative chemotherapy led to a higher 5-year overall survival rate versus surgery alone (38% versus 24%, respectively; HR 0.69; 95% CI 0.50–0.95, P = 0.02) [ 20 ]. Recently, the randomized phase II/III FLOT4-AIO study compared perioperative FLOT regimen (fluorouracil, leucovorin, oxaliplatin, and docetaxel) with previous standard ECF/ECX (epirubicin, cisplatin, and fluorouracil/capecitabine) regimen in gastric or GEJ cancer patients who had cT2 or higher and nodal positive (cN +) disease [ 21 ]. The results suggested that the FLOT regimen could improve overall survival (50 months versus 35 months), confirming the role of the FLOT regimen as the new standard perioperative treatment for resectable gastric cancer [ 5 , 18 ].
Since most of the clinical trials mentioned above were conducted in western countries, these perioperative regimens (ECF, CF, and FLOT) are less frequently used in Asia. In the phase III PRODIGY trial, 530 Korean patients with cT2-3N + or cT4N any gastric or GEJ cancer were randomly randomized to the neoadjuvant or adjuvant group. Patients in the neoadjuvant arm underwent preoperative DOS (docetaxel, oxaliplatin, and S-1) followed by surgery and S-1 adjuvant chemotherapy, while those in the adjuvant arm received upfront radical surgery followed by S-1 chemotherapy [ 22 ]. The perioperative chemotherapy group had significantly higher rates of R0 resection and pathological complete response (pCR) (95% and 10.4%, respectively). Moreover, PFS was improved in the perioperative arm compared to the adjuvant arm (HR 0.70; 95% CI 0.52–0.95; P = 0.023). The major criticism of this study was that the adjuvant S-1 monotherapy was insufficient for stage III patients, considering another phase III study had demonstrated the superiority of docetaxel plus S-1 to S-1 for 3-year relapse-free survival (RFS) in stage III gastric cancer [ 23 ]. Recently, the phase III RESOLVE trial conducted in China investigated the role of perioperative S-1 plus oxaliplatin (SOX) chemotherapy versus upfront surgery followed by adjuvant chemotherapy [ 24 ]. This study recruited over 1,000 patients with cT4aN + or cT4bN any gastric or GEJ adenocarcinoma, of whom over 60% had gastric cancer. Patients in the intervention group received perioperative SOX (three preoperative cycles and five postoperative cycles followed by three cycles of S-1 monotherapy). The two adjuvant groups received surgery followed by SOX or CAPOX (capecitabine and oxaliplatin) chemotherapy. These results suggested that the perioperative SOX chemotherapy could improve the 3-year disease-free survival (DFS) rate compared to adjuvant CAPOX therapy (59.4% vs. 51.1%, respectively, P = 0.028).
Based on the evidence shown above, perioperative chemotherapy has become the standard treatment in many countries. The FLOT regimen is the most commonly used in Western countries according to the evidence from the FLOT4-AIO study[ 21 ], while the SOX regimen is more recommended in China, based on the results of the RESOLVE study[ 24 ]. However, perioperative chemotherapy is less recommended in Japan, since evidence of the superiority of neoadjuvant chemotherapy is still lacking among Japanese patients[ 25 ].
Adjuvant chemotherapy
Adjuvant chemotherapy is recommended for patients who undergo primary surgery and have stage II or stage III disease due to improvement in survival demonstrated by several clinical trials, particularly in Asian patients. The multi-center phase III CLASSIC trial undertaken in South Korea, China, and Taiwan compared upfront D2 surgery followed by CAPOX adjuvant chemotherapy versus D2 gastrectomy alone in patients with stage II-IIIB gastric cancer [ 26 , 27 ]. Adjuvant CAPOX chemotherapy significantly improved both 5-year DFS (68% vs. 53%; HR 0.58; 95% CI, 0.47 to 0.72; P < 0.0001) and OS (78% vs. 69%; HR 0.66; 95% CI, 0.51 to 0.85; P = 0.0015) compared with surgery alone. Another similar phase III ACTS-GC trial from Japan randomly assigned 1,059 stage II or III GC patients to undergo D2 surgery followed by S-1 monotherapy or D2 surgery alone and showed that adjuvant S-1 monotherapy for one year led to a better 3-year OS than surgery alone (80.1% vs. 70.1%; HR 0.68; 95% CI, 0.52 to 0.87; P = 0.003). The survival benefit persisted after five years of follow-up [ 28 ]. Moreover, the phase III JACCRO GC-07 trial investigated the superiority of adjuvant docetaxel plus S-1 over S-1 monotherapy for pathological stage III gastric cancer [ 23 ]. The addition of docetaxel to S-1 after surgery showed a better 3-year RFS (66% vs. 50%; HR 0.632; 99.99% CI, 0.400 to 0.998; P < 0.001) in the second interim analysis, and the study was terminated as recommended by the independent data and safety monitoring committee. The RESOLVE trial also investigated the non-inferiority of adjuvant SOX chemotherapy compared with the CAPOX regimen. The 3-year DFS was statistically comparable between the two groups (56.5% vs. 51.1%; HR 0.86; 95% CI, 0.68 to 1.07; P = 0.17) [ 24 ]. Based on the results of the phase III trials presented above, several cytotoxic regimens could be used as adjuvant treatments for stage II-III GC after radical surgery, including S-1, CAPOX, SOX, and DS. The choice of regimens depends on many factors, including the pathological staging, patient performance status, and toxicity profile. In general, S-1 monotherapy is more recommended for stage II disease or for patients with poor performance status. Combination therapies such as CAPOX, SOX, or DS are often recommended for pathological stage III disease[ 17 , 25 ].
GC with microsatellite instability-high (MSI-H) or mismatch-repair deficiency (dMMR) is a distinct subtype [ 11 ]. Recently, an individual-patient-data meta-analysis including data from four large phase III studies (CLASSIC, ARTIST, MAGIC, and ITACA-S trial) explored the role of adjuvant chemotherapy in the MSI-H subtype [ 29 ]. It showed that for resectable MSI-H/dMMR GC patients, the prognosis of patients who received surgery alone was better than those who underwent surgery followed by adjuvant chemotherapy, even though the sample size of MSI-H/dMMR in this meta-analysis was very modest (N = 121). Based on this result, adjuvant chemotherapy is not recommended for resectable MSI-H/dMMR GC patients in the latest ESMO guideline [ 5 ]. Additionally, the updated CSCO guidelines suggest that either observation or adjuvant chemotherapy could be considered after a thorough discussion with the patients regarding the possible risks and benefits [ 17 ].
Adjuvant chemoradiotherapy
Unlike chemotherapy, the role of radiotherapy for resectable GC in the adjuvant setting is controversial. Adjuvant chemoradiotherapy (CRT) was once adopted in North America, according to the results of the phase III INT-0116 trial [ 30 ]. In this study, 556 patients with resectable GC or GEJ adenocarcinoma were randomly assigned to the upfront surgery plus adjuvant CRT group or the surgery group. Patients in the experimental arm received adjuvant fluorouracil chemotherapy plus 4500 cGy of radiation (5 × 5). Overall, CRT did prolong the OS compared to surgery alone (36 vs. 27 months, respectively; P = 0.005). However, most patients in this study received D0 or D1 lymphadenectomy and only 10% had D2 lymphadenectomy. The extent of dissection might affect the outcome of the surgery-only group. The phase III ARTIST trial from Korea evaluated the role of postoperative CRT based on the D2 dissection backbone [ 31 ]. Four hundred fifty-eight patients who received D2 lymphadenectomy and R0 resection were enrolled and randomly assigned to the adjuvant chemotherapy arm (capecitabine plus cisplatin, XP) or the adjuvant CRT arm (XP-XRT-XP). Unfortunately, the addition of radiotherapy postoperatively did not improve their DFS ( P = 0.0862). However, in the subgroup analysis, DFS was significantly prolonged in the CRT arm in the patients with lymph node-positive (N +) disease (3-year DFS rate: 77.5% vs.72.3%, HR 0.69, 95% CI 0.474–0.995, P = 0.0365). Based on these findings, the subsequent ARTIST II trial further explored the role of adjuvant CRT in patients with lymph node-positive GC [ 32 ]. Five hundred forty-six patients after D2 dissection were randomly assigned to adjuvant S-1, adjuvant SOX, and adjuvant SOX plus radiotherapy (SOXRT) in a 1:1:1 ratio. However, there was no significant difference in DFS between the adjuvant SOX and SOXRT treatments (3-year DFS rate: 72.8% vs.74.3%; HR 0.97, 95% CI 0.66–1.42, P = 0.879). Therefore, according to current results from these clinical trials, adjuvant CRT is not recommended in patients who received D2 lymphadenectomy and R0 resection.
Novel perioperative therapies
Perioperative targeted therapy.
Anti-HER2 and anti-vascular endothelial growth factor (VEGF) therapies have been recommended as the standard treatments for advanced GC in the first- and second-line setting, respectively. However, the role of targeted therapy in the perioperative or adjuvant setting is still unclear and is currently under investigation.
Anti-HER2 therapy
According to the ToGA study, adding trastuzumab to chemotherapy improved the OS in patients with metastatic HER2-positive GC [ 14 ]. However, the role of anti-HER2 therapy in resectable GC was unclear. In the multicenter phase II HER-FLOT study, patients with HER2-positive esophagogastric adenocarcinoma received perioperative FLOT chemotherapy for four cycles preoperatively and four cycles postoperatively, followed by 9 cycles of trastuzumab monotherapy [ 33 ]. The pCR rate was 21.4%, and the median DFS was 42.5 months. The phase II randomized PETRARCA study investigated the efficacy of adding trastuzumab and pertuzumab to perioperative FLOT chemotherapy in patients with ≥ cT2 or cN + resectable GC [ 34 ]. The pCR rate was significantly improved with trastuzumab and pertuzumab (35% vs. 12%, P = 0.02), and the R0 resection rate and surgical morbidity were comparable between both groups. However, adding targeted therapy to perioperative chemotherapy did not improve DFS or OS and caused more severe adverse events (≥ grade 3), especially diarrhea (41% vs. 5%) and leukopenia (23% vs. 13%). Based on these results, the trial did not proceed to phase III. Another phase II NEOHX study recruited 36 HER2-positive GC patients who received perioperative CAPOX plus trastuzumab treatment, followed by 12 cycles of trastuzumab maintenance therapy [ 35 ]. The pCR rate, 18-month DFS rate, and 5-year OS rate were 9.6%, 71%, and 58%, respectively. The randomized phase II INNOVATION trial assigned patients to 3 groups: perioperative chemotherapy, chemotherapy plus trastuzumab, and chemotherapy plus trastuzumab and pertuzumab [ 36 ]. According to the investigators' choice, the chemotherapy could be FLOT, CAPOX, FOLFOX, or XP. The primary endpoint was major pathological response (MPR) rate, and the result is pending. In general, adding anti-HER2 therapy to chemotherapy showed certain efficacy in the perioperative setting, but the associated survival benefit should be further investigated in a larger randomized trial.
Anti-VEGF therapy
As for anti-VEGF therapy, the randomized, open-label, phase II/III ST03 trial recruited 1,063 resectable esophagogastric adenocarcinoma patients and randomly assigned them to perioperative chemotherapy (ECX) group or perioperative chemotherapy plus bevacizumab group [ 37 ]. The result showed that adding bevacizumab did not improve the 3-year OS (48.1% vs. 50.3% for chemotherapy alone; HR 1.08; 95% CI, 0.91 to 1.29; P = 0.36). Besides, adding bevacizumab was associated with higher rates of postoperative anastomotic leak (24% vs. 10%). Ramucirumab, a VEGF receptor 2 inhibitor, has become one of the standard choices in the second-line treatment of GC [ 5 , 17 , 18 ]. The RAMSES/FLOT7 evaluated the efficacy of adding ramucirumab to perioperative FLOT for resectable GC [ 38 ]. The R0 resection rate in the intervention group was improved compared to the chemotherapy group (96% vs. 82%, P = 0.0093). The median DFS was prolonged in the FLOT plus ramucirumab group (32 months vs. 21 months), while the OS was similar in both groups (46 months vs. 45 months).
Perioperative immunotherapy
Based on several phase III clinical trials, programmed death 1 (PD-1) inhibitors were approved for first- and third-line treatment of unresectable/metastatic GC in different countries [ 5 , 17 , 18 ]. However, the role of ICI in resectable GC remains unclear and is being investigated in various clinical trials. In the randomized phase II DANTE trial, patients with resectable GC were assigned to the experimental arm with the PD-L1 inhibitor atezolizumab plus FLOT chemotherapy and the control arm with standard FLOT chemotherapy [ 39 ]. The R0 resection rate, surgical morbidity and mortality were comparable in both groups. Atezolizumab combined with chemotherapy was associated with tumor downstage and pathological regression, which were more pronounced in patients with a higher PD-L1 combined positive score (CPS).
Several single-arm phase II clinical trials explored the efficacy of perioperative ICIs combined with different treatments (chemotherapy, targeted therapy, or radiotherapy) in resectable GC [ 40 , 41 , 42 , 43 , 44 ]. The pCR rates ranged from 10 to 41%. In the phase III ATTRACTION-5 trial (NCT03006705), the use of nivolumab in the adjuvant setting was investigated. Patients who have undergone D2 surgery will receive either S-1 for one year or CAPOX for six months, with nivolumab added to the adjuvant therapy in the intervention arm. The primary endpoint of the study is relapse-free survival (RFS). The result was announced recently. Unfortunately, the addition of nivolumab did not extend the RFS compared with adjuvant chemotherapy alone. Additionally, the role of pembrolizumab in combination with perioperative chemotherapy for resectable GC is being examined in the phase III clinical trial, KEYNOTE-585 [ 45 ]. The chemotherapy regimens under investigation are XP, FP, or FLOT, and the primary endpoints of the study are OS, event-free survival (EFS), and pCR rate. The potential survival benefits and efficacy of ICI are also being evaluated in the double-blind, randomized phase III MATTERHORN study, which is based on the FLOT backbone [ 46 ]. Patients with resectable GC will receive either perioperative FLOT or FLOT plus durvalumab (a PD-L1 antibody). The primary endpoint of the study is EFS, with secondary endpoints including OS and pCR rate.
For the dMMR/MSI-H subgroup, as discussed above, the value of chemotherapy was controversial. Considering dMMR/MSI-H is a predictive biomarker for immunotherapy in advanced GC, treatment with immune checkpoint inhibitors in the perioperative setting has the potential to improve the response rate and survival. The phase II GERCOR NEONIPIGA study evaluated the response rate and safety of the combination of neoadjuvant nivolumab and low-dose ipilimumab followed by adjuvant nivolumab in patients with dMMR/MSI-H locally advanced G/GEJ adenocarcinoma. Among 29 patients who underwent surgery, 17 (58.6%; 90% CI, 41.8–74.1) achieved pCR[ 47 ]. Similarly, the pCR rate of tremelimumab plus durvalumab was 60% in the neoadjuvant setting (cohort 1) in the phase II INFINITY study[ 48 ]. Based on these encouraging results, it is possible for patients who achieved pCR after neoadjuvant immunotherapy to avoid surgery. Cohort 2 of the INFINITY study has started enrollment to investigate the activity of tremelimumab plus durvalumab as the definitive treatment for dMMR/MSI-H locally advanced GC.
Management for unresectable/metastatic GC
Chemotherapy.
Cytotoxic agents, including fluoropyrimidine, platinum, taxanes and irinotecan, are the main treatment in advanced gastric cancer. Generally, fluoropyrimidine (fluorouracil, capecitabine, and S-1) combined with platinum is used as the backbone therapy in the first line. Oxaliplatin is considered to be as effective as cisplatin. In the phase III SOX-GC trial, the SOX regimen showed improved survival compared to the SP regimen in diffuse or mixed-type GC[ 49 ]. For patients who are not fit for intensive chemotherapy (older age or poor performance status), the phase III GO2 trial showed that a modified dose of two-drug chemotherapy (60% of the full dose) provided a better tolerance but did not compromise the clinical outcome[ 50 ]. Paclitaxel, docetaxel, and irinotecan are commonly used in the second line of chemotherapy. In the ABSOLUTE phase III clinical trial conducted in Japan, weekly use of albumin-bound paclitaxel (nab-paclitaxel) was not inferior to weekly solvent-based paclitaxel in terms of overall survival[ 51 ]. In third-line treatment, trifluridine-tipiracil (TAS-102), an oral cytotoxic agent, has been proven in the phase III TAGS trial to improve overall survival compared with placebo (5.7 vs.3.6 months, HR 0.69, 95% CI 0.56–0.85)[ 52 ].
Immune Checkpoint Inhibitors (ICIs) in unresectable/metastatic GC
Immune checkpoint inhibitors (ICIs) (monotherapy or combined with other treatments) have shown anti-tumor effects across a spectrum of solid tumors, including gastrointestinal tumors. Here, we present an overview of current evidence of ICI treatment in GC (Table 2 ) and discuss different predictive biomarkers for ICIs.
KEYNOTE-062 was the first global, randomized phase III trial to compare the efficacy and safety of immuno-monotherapy (pembrolizumab) or immunotherapy plus chemotherapy versus standard chemotherapy in HER2-negative advanced GC in the first-line setting [ 53 ]. According to the last update in ASCO 2022, it was suggested that pembrolizumab monotherapy was non-inferior to chemotherapy alone (cisplatin and fluorouracil/capecitabine) in patients with PD-L1 CPS ≥ 1 (median OS 10.6 vs. 11.1 months, HR 0.90, 95% CI 0.75–1.08) but was superior in the CPS ≥ 10 population (median OS 17.4 vs. 10.8 months; HR, 0.62; 95% CI, 0.45–0.86) [ 54 ]. However, the combination of pembrolizumab and chemotherapy did not bring OS benefit compared to chemotherapy alone in either CPS ≥ 1 (12.5 vs. 11.1 months; HR, 0.85; 95% CI, 0.71–1.02) or CPS ≥ 10 (12.3 vs. 10.8 months; HR, 0.76; 95% CI, 0.56–1.03) subgroup [ 54 ]. In another double-blind, placebo-controlled phase III KEYNOTE-859 study, the addition of pembrolizumab to chemotherapy (FP or CAPOX) demonstrated slight survival benefit compared with chemotherapy alone (OS 12.9 vs. 11.5 months, HR, 0.78; 95% CI, 0.70–0.87. PFS 6.9 vs. 5.6 months, HR, 0.76; 95% CI, 0.67–0.85). The results were generally consistent in different PD-L1 CPS subgroups[ 55 ].
CheckMate-649 is another global, randomized, phase III trial investigating the effects of ICIs (nivolumab plus ipilimumab, a CTLA-4 inhibitor) or ICI (nivolumab) plus chemotherapy versus chemotherapy (CAPOX or FOLFOX) alone in metastatic HER2-negative GC patients [ 56 ]. One thousand five hundred eighty-one patients were assigned to nivolumab plus chemotherapy arm or chemotherapy arm. The addition of nivolumab to chemotherapy improved the OS (14.4 vs. 11.1 months; HR 0.71; 98.4% CI, 0.59 to 0.86; P < 0.0001) and PFS (7.7 vs. 6.05 months; HR 0.68; 98% CI, 0.56 to 0.81; P < 0.0001) for the patients with PD-L1 CPS ≥ 5; therefore both primary endpoints were met. For all-randomized patients, nivolumab combined with chemotherapy also improved OS (13.8 vs. 11.6 months; HR 0.80; 99.3% CI, 0.68 to 0.94; P = 0.0002). Moreover, all CPS subgroups exhibited an increased objective response rate in the nivo-chemotherapy arm. However, the chemo-free treatment with nivolumab and ipilimumab did not show OS improvement compared to chemotherapy alone [ 57 ]. Based on these findings, nivolumab combined with chemotherapy was listed as one of the recommended first-line treatments in the NCCN, ESMO, and CSCO guidelines [ 5 , 17 , 18 ].
ATTRACTION-04 was a randomized, double-blind, placebo-controlled, multicenter phase II/III trial that evaluated the effects of nivolumab plus chemotherapy (SOX or CAPOX) compared with chemotherapy alone in the first-line treatment for HER2-negative advanced GC in the Asian population, regardless of PD-L1 expression [ 58 ]. The combination therapy significantly improved the PFS (HR 0·68; 98·51% CI 0·51–0·90; P = 0·0007) but not the OS (both groups achieved > 17 months of median OS). One of the possible reasons for the different results of OS between ATTRACTION-04 and CheckMate-649 could be the subsequent anticancer therapies, whereby the proportion of patients who received subsequent anticancer treatments or ICIs therapy was much higher in ATTRACTION-04 (66% vs. 39% in CheckMate-649).
The efficacy of immunotherapy plus chemotherapy was further confirmed in the Asian phase III ORIENT-16 trial, which compared sintilimab plus chemotherapy (CAPOX) to chemotherapy alone as the first-line treatment [ 59 ]. The pre-specified interim result was reported at ESMO 2021. Sintilimab plus chemotherapy showed a survival benefit versus chemotherapy alone in patients with CPS ≥ 5 (18.4 vs. 12.9 months; HR 0.660; 95% CI 0.505–0.864) and all randomized patients (15.2 vs. 12.3 months; HR 0.766; 95% CI 0.626–0.936). Another PD-1 antibody, tislelizumab, is currently being investigated in the phase III RATIONALE-305 trial [ 60 ]. Advanced GC patients are randomized to receive tislelizumab plus chemotherapy (CAPOX/FP regimen) or chemotherapy alone. The primary endpoints are PFS and OS. Results from the interim analysis of the PD-L1 + (i.e., PD-L1 TAP score ≥ 5%) population were represented at 2023 ASCO-GI, showing that tislelizumab plus chemotherapy led to significant OS (17.2 vs. 12.6 months; HR 0·74; 95% CI 0·59–0·94) and PFS (7.2 vs. 5.9 months; HR 0·67; 95% CI 0·55–0·83) improvement compared to chemotherapy alone[ 61 ]. The ITT population outcomes will be reported after the final analysis.
In summary, in first-line treatment for HER2-negative advanced GC, the addition of anti-PD-1 therapy could improve clinical outcomes in patients with high PD-L1 expression, according to the results from CheckMate-649, ORIENT-16, and RATIONALE-305. For patients with low PD-L1 expression or unknown PD-L1 status, the survival benefit of adding PD-1 antibodies is still controversial (discussed below), and the risk–benefit balance of ICIs treatment should be considered, and decisions should be discussed case by case.
The role of maintenance therapy with ICIs after first-line chemotherapy was evaluated in the phase III JAVELIN Gastric 100 trial [ 62 ]. Patients with HER2-negative advanced GC without progression after at least 12 weeks of first-line chemotherapy (oxaliplatin plus fluoropyrimidine) were randomly assigned to avelumab (a PD-L1 inhibitor) maintenance or continued chemotherapy. Avelumab maintenance did not show OS benefit compared to chemotherapy (24-month OS rate: 22.1% versus 15.5%; HR 0.91; 95% CI, 0.74–1.11; P = 0.1779).
Second line and beyond
The randomized, open-label, phase III KEYNOTE-061 trial compared pembrolizumab monotherapy with paclitaxel in patients with advanced GC or GEJ cancer in the second-line setting [ 53 ]. Though the primary endpoints (the OS and PFS in patients with PD-L1 CPS ≥ 1) were not met, it was suggested that the efficacy of pembrolizumab monotherapy was associated with the PD-L1 CPS level. Patients with CPS ≥ 10 had a better outcome in the pembrolizumab group than in the chemotherapy group.
KEYNOTE-059 was a phase II study that explored the effect of pembrolizumab in patients with advanced GC after progression from ≥ 2 lines of treatment [ 63 ]. Among the 259 patients enrolled, the ORR and median duration of response (DoR) was 11.6% and 8.4 months, respectively. Moreover, pembrolizumab showed higher efficacy in the subgroup with PD-L1-positive cancer (CPS ≥ 1) compared to PD-L1-negative cancers (ORR 15.5% vs. 6.4%; DoR 16.3 vs. 6.9 months, respectively). The phase III ATTRACTION-2 study compared nivolumab monotherapy versus placebo in advanced GC patients after two lines of therapy, regardless of the PD-L1 expression [ 64 ], and survival benefit was observed in the nivolumab group (OS 5.3 vs. 4.1 months; HR 0·63, 95% CI 0·51–0·78; P < 0·0001). Based on the results of this study, nivolumab is recommended as monotherapy in third-line treatment for GC in the CSCO guideline but not in the ESMO or NCCN guidelines due to the patients enrolled being exclusively Asian. The role of avelumab in the third-line treatment for advanced GC was investigated in the phase III JAVELIN Gastric 300 trial [ 65 ]. Though avelumab showed a more manageable safety than the physician's choice of chemotherapy, it did not improve OS (primary endpoint, 4.6 vs. 5.0 months; HR 1.1, 95% CI 0·9–1.4; P = 0.81), PFS, or ORR.
Molecular Biomarkers of Immunotherapy in GC
HER2-positive GC, defined as immunohistochemical (IHC) expression 3+ or 2 + combined with positive fluorescent in situ hybridization (FISH) verification, accounts for approximately 15–20% of gastric or gastroesophageal cancer. The phase III ToGA study has established trastuzumab combined with chemotherapy as the standard first-line treatment for HER2-positive advanced GC [ 14 ]. In preclinical models, HER2 signaling could regulate the recruitment and activation of tumor-infiltrating immune cells [ 66 ]. Besides, trastuzumab has been shown to upregulate the expression of PD-1 and PD-L1 [ 67 , 68 ], and anti-PD-1 antibodies could significantly increase the therapeutic activity of HER2 inhibitors [ 69 ]. Several phase I/II studies demonstrated the promising efficacy of the addition of ICIs to trastuzumab and chemotherapy in HER2-positive GC. In the phase Ib Ni-HIGH study conducted in Japan, patients with HER2-positive advanced GC received nivolumab, trastuzumab, and chemotherapy (CAPOX or SOX regimen) in the first-line setting, and the ORR was 75%, as reported at ASCO 2020 [ 70 ]. The multi-institutional phase Ib/II PANTHERA trial explored the efficacy and safety of the combination of pembrolizumab, trastuzumab and chemotherapy as first-line therapy for HER2-positive advanced GC [ 71 ]. The updated data at ASCO-GI 2021 showed that the ORR was 76.7% (CR 16.3%, PR 60.5%), the PFS was 8.6 months (95% CI 7.2–16.5 months), and the OS was 19.3 months (95% CI 16.5-NR). The striking efficacy was also reported in another phase II study, in which patients with HER2-positive GC received pembrolizumab, trastuzumab and chemotherapy (oxaliplatin/cisplatin + capecitabine/5-FU) [ 72 ]. Overall, the ORR was 91% and DCR was 100%. The median PFS and OS was 13·0 months and 27·3 months, respectively, which was much better than the OS reported in the ToGA study. Recently, the randomized, double-blind, placebo-controlled phase III KEYNOTE-811 trial reported the results of its first interim analysis [ 73 ], in which patients with metastatic HER2-positive GC or GEJ cancer received pembrolizumab or placebo plus trastuzumab and chemotherapy. The results showed that adding pembrolizumab to trastuzumab and chemotherapy could markedly increase the ORR (74.4% vs. 51.9%; the estimated difference between the two groups was 22.7%; 95% CI, 11.2–33.7%; P = 0.00006). Based on this result, the FDA approved pembrolizumab combined with trastuzumab and chemotherapy as the first-line treatment for advanced HER2-positive gastric or GEJ adenocarcinoma. The results of the primary endpoints (PFS and OS) are still immature.
MSI-H tumor is one of the four subtypes of GC according to The Cancer Genome Atlas (TCGA) Research Network [ 11 ]. The incidence of MSI-H status in GC was reported to range from 8 to 25%, which was much lower in metastatic disease [ 74 ]. Mismatch repair (MMR) proteins are supposed to fix the errors that occur during DNA replication. When MMR proteins are deficient, the defects of DNA replication will lead to the accumulation of mutations and the expression of neoantigens, which may act as potential targets of immune cells [ 75 ]. Hence, it is reasonable that tumors with MSI-H/dMMR status may attract more immune cell infiltration and enhance the effect of immune checkpoint inhibitors. A post hoc analysis of KEYNOTE-059 (third-line treatment), KEYNOTE-061 (second-line treatment), and KEYNOTE-062 (first-line treatment) was conducted to evaluate the efficacy of pembrolizumab versus chemotherapy in the patients with MSI-H advanced G/GEJ adenocarcinoma [ 15 ]. Overall, 7 of 174 patients (4.0%) in KEYNOTE-059, 27 of 514 (5.3%) in KEYNOTE-061, and 50 of 682 (7.3%) in KEYNOTE-062 with MSI-H status were enrolled. By the time of analysis, the OS of the patients with MSI-H was not reached for pembrolizumab monotherapy in KEYNOTE-059, 061 and 062, or for pembrolizumab combined with chemotherapy in KEYNOTE-062, compared with an OS of around 8 months for chemotherapy alone. Besides, the ORR was much higher in the immunotherapy groups. In another meta-analysis including four phase III trials (KEYNOTE-062, CheckMate-649, JAVELIN Gastric 100, and KEYNOTE-061), 2545 patients with known MSI status were enrolled, and the proportion of MSI-H was 4.8% [ 76 ]. In the MSI-H group, the HR for OS benefit with immunotherapy was 0.34 (95% CI 0.21–0.54), compared to 0.85 (95% CI 0.71–1.00) for the MSS group. Among the patients with MSI-H status, the HR for PFS was 0.57 (95% CI 0.33–0.97; P = 0.04), and the odds ratio (OR) for ORR was 1.76 (95% CI 1.10–2.83; P = 0.02). Altogether, these findings suggested that MSI-H status was a predictive biomarker for immune checkpoint inhibitor treatments, regardless of the line of therapy.
Epstein-Barr virus-associated GC (EBVaGC) is another distinct molecular subtype of the TCGA classification [ 11 ], accounting for about 9% of GC in the TCGA cohort and approximately 5% in China [ 77 , 78 ]. EBV has been linked to CD8 + T cell infiltration and increased expression of PD-L1 and PD-L2 [ 11 , 79 ], making it a potential biomarker for ICI treatment. While a Korean study with a small sample size (n = 6) once reported a 100% response rate in EBV-positive advanced GC [ 80 ], several other studies did not demonstrate a high response rate [ 81 , 82 , 83 ]. Differences in response rates across studies may be attributed to confounding factors such as tumor mutational burden (TMB) and PD-L1 expression. Therefore, the role of EBV positivity in immunotherapy for GC remains unclear and requires further investigation.
As discussed earlier, the level of PL-L1 expression, especially the CPS score, has been considered a predictive biomarker for response to ICIs. However, the reliable cut-off value to predict the benefit of immunotherapy is needed to be determined. The cut-off points often used in clinical trials are 1, 5 and 10. In the KEYNOTE-059 trial, CPS ≥ 1 was used to separate the patients that could benefit from third-line pembrolizumab treatment [ 63 ]. However, this benefit was not seen compared to chemotherapy in the KEYNOTE-061/062 trials [ 53 , 84 ]. In KEYNOTE-061/062, CPS ≥ 10 effectively differentiated the response to pembrolizumab. Patients with CPS ≥ 10 had better OS benefits than those with CPS ≥ 1. A comprehensive analysis of patients with CPS ≥ 10 in KEYNOTE-059, 061 and 062 also showed consistent improvement toward better outcomes with pembrolizumab in different lines of treatment in this subgroup [ 85 ]. In the CheckMate-649 and ORIENT-16 studies, CPS ≥ 5 was used as the cut-off value for the primary endpoint OS. Though the OS benefit of nivolumab plus chemotherapy was also observed in all randomized patients in CheckMate-649, the subgroup analysis suggested that the benefit was insignificant in the CPS < 5 or < 1 group [ 86 ]. A recent study reconstructed unreported Kaplan–Meier plots of PD-L1 CPS subgroups of three phase III trials (CheckMate-649, KEYNOTE-062, and KEYNOTE-590) and investigated the outcome of low CPS subgroup [ 87 ]. The result suggested that patients with low PD-L1 expression (CPS 1–4 and CPS 1–9) did not benefit from adding ICIs to chemotherapy. In summary, although the predictive role of PD-L1 CPS for immunotherapy efficacy has been demonstrated in multiple clinical trials, there is still a need to determine the optimal cut-off value for CPS and to develop further classifications for patients with low CPS scores. Recently, the result of the phase III RATIONALE-305 trial suggested that the TAP score > 5% also had predictive value for ICI treatment in gastric cancer[ 61 ], and further exploration is needed.
Tumor mutation burden (TMB)
It is hypothesized that a high TMB status results in the high expression of neoantigens, which are immunogenic and can induce the response of the immune system and potentially increase the efficacy of ICI treatment. In a phase Ib/II study that explored the efficacy of the PD-1 antibody toripalimab in patients with advanced GC, patients with TMB-high (TMB-H, TMB ≥ 12 mut/Mb) showed a higher ORR and better OS compared with patients with TMB-L status (ORR 33.3% vs. 7.1%, P = 0.017; OS 14.6 vs. 4.0 months, P = 0.038)[ 88 ]. In the subgroup analysis of the KEYNOTE-061 study, the TMB status (≥ 10 or < 10 mut/Mb) was associated with response rate, PFS, and OS in patients treated with pembrolizumab. In the TMB-H subgroup, pembrolizumab demonstrated a better OS compared with paclitaxel, and this benefit remained even when MSI-H patients were excluded[ 89 ]. Though FDA granted approval for the use of pembrolizumab in patients with TMB-H (i.e., TMB ≥ 10 mutations/Mb) advanced solid tumors that progressed after standard treatments, according to the subgroup analysis of KEYNOTE-158 study[ 90 ], the evidence is still not enough for the use of ICIs in TMB-H gastric cancer, and phase III studies to illustrate the predictive value of TMB are needed.
Molecular targeted therapy in unresectable/metastatic GC
Molecular targeted therapy remains an essential treatment option for patients with advanced GC, aimed to inhibit tumor proliferation and increase survival rates. Targeted therapies, including anti-HER2, anti-angiogenesis, and other biomarker-directed therapies, have demonstrated promising efficacy in treating GC, with significant benefits observed in biomarker-enriched patients (Table 3 ). Therefore, next-generation sequencing or ctDNA detection is crucial for mGC patients to establish a comprehensive molecular profile, including the status of HER2, fibroblast growth factor receptor (FGFR), Claudin18.2 (CLDN18.2), PD-L1 and EGFR.
HER2, also known as ERBB2, is a member of the ERBB protein families that includes the epidermal growth factor receptor (EGFR or HER1), HER3, and HER4 [ 91 ]. HER2 overexpression or amplification has been found in a range of 7.3% to 20.2% in advanced gastric and gastroesophageal junction adenocarcinomas, with the overexpression rate varying globally [ 92 ]. In addition, intestinal-type gastric cancers and those arising from the proximal stomach or gastroesophageal junction are more likely to exhibit HER2 positivity. [ 11 , 93 ].
Trastuzumab is a humanized monoclonal antibody that targets HER2 extracellular domain 4, then inhibits downstream signal activation and cancer cell proliferation. Trastuzumab plus chemotherapy has been established as the standard first-line treatment for HER2-positive advanced GC. The landmark ToGA trial revealed that trastuzumab plus chemotherapy significantly improved the overall survival of patients with advanced GC [ 14 ], especially for patients with HER2 positivity, who were identified as having HER2 immunohistochemistry (IHC) scores of 2 + and fluorescence in situ hybridization (FISH)-positive or HER2 IHC 3 + based on a post-hoc exploratory analysis [ 92 ]. The EVIDENCE trial has demonstrated that combining first-line trastuzumab with chemotherapy was associated with improved clinical outcomes in Chinese patients with HER2-positive metastatic GC, providing real-world evidence. [ 94 ].
However, subsequent attempts of HER2-targeted therapy in advanced GC were not as successful as expected. Even though pertuzumab [ 95 , 96 ], trastuzumab emtansine (T-DM1) [ 97 ], and lapatinib [ 98 , 99 ] were all investigated in several first-line and second-line trials, no survival improvement was observed in any of these trials. Additionally, trastuzumab beyond progression also failed to show a survival benefit in pre-treated HER2-positive GC patients in the T-ACT trial [ 100 ].
Potential resistance mechanisms of HER2-targeted therapy
Primary or acquired resistance is a major impediment to the management of mGC patients, while mechanisms underlying the poor efficacy of HER2-directed therapy in GC are not fully understood. Multiple potential resistance mechanisms have been researched, as listed below, and further studies are warranted to improve treatment resistance in GC patients treated with HER2-targeted therapy in clinical settings.
HER2 heterogeneity
Intratumoral HER2 heterogeneity is observed in 23% to 79% of GC patients and is associated with patients’ survival [ 101 , 102 , 103 ]. Specifically, Shusuke et al. reported prolonged survival in homo-HER2 positive GC patients, defined as all tumor cells overexpressing HER2 in biopsy specimens [ 101 ]. Tumor cells with HER2 overexpression or amplification are killed during HER2-targeted therapy, while residual drug-resistant colonies keep proliferating and eventually take control, leading to tumor recurrence. As a result, resistance to HER2-targeted therapy has been associated with the heterogeneity of HER2 expression [ 101 , 104 , 105 ]. Discordance between next-generation sequencing and FISH/IHC may also indicate intratumoral heterogeneity and result in an unfavorable treatment outcome. In addition, there still exist discrepancies in HER2 status between primary tumor and metastatic sites, which increases the risk of HER2-targeted therapy failure due to false-positive HER2 detection [ 106 , 107 ].
Loss of HER2 expression
For mGC patients experiencing progression on trastuzumab, 29–69% of them may experience loss of HER2 expression, which is an important factor responsible for resistance [ 108 , 109 , 110 ]. Given the risk of HER2 expression loss during treatment, patients should re-evaluate HER2 status upon progression after anti-HER2 therapy to determine the most optimal treatment.
Gene amplification
Receptor tyrosine kinase (RTK) amplification was commonly detected in MET-amplified metastatic GC, with 40% to 50% of cases exhibiting co-amplification of either HER2 or EGFR. These patients did not usually respond to HER2-targeted therapy, but MET and HER2 combination inhibition could sometimes bring extra clinical benefit [ 111 ]. CCNE1, which encodes the cell cycle regulator cyclin E1, is another oncogene co-amplified with HER2 in metastatic GC. CCNE1 co-amplification has been found to be more strongly related to HER2-positive AGC than to HER2-positive breast cancer [ 112 ]. In a phase II study of lapatinib with capecitabine and oxaliplatin in HER2-positive AGC patients, CCNE1 amplification was demonstrated to play a role in resistance to HER2-targeted therapy [ 113 ]. A high level of copy number variation for CCNE1 has also been associated with worse survival in patients with HER2-positive metastatic GC treated with trastuzumab [ 114 ]. Other studies have also reported that deletion of ErbB2 16 exon and co-mutation and/or amplification of KRAS, HER3, EGFR, PI3K or PTEN could contribute to the resistance of anti-HER2 therapy [ 109 , 113 , 115 , 116 ].
Alterations in intracellular signaling
HER2-targeted therapy suppresses downstream signaling pathways by blocking the binding of HER2 receptors and ligands, which inhibits the migration and proliferation of tumor cells and leads to apoptosis. RTK/RAS/PI3K signaling alterations have been shown to be involved in the development of resistance to trastuzumab. [ 109 ]. Furthermore, activation of the bypass pathway might also result in resistance. Sampera et al. discovered that SRC-mediated persistent activation of the MAPK-ERK and PI3K-mTOR pathways was connected to the treatment resistance in HER2-positive GC cell lines [ 117 ]. NRF2 has also been associated with HER2 resistance by activating the PI3K-mTOR signaling pathway [ 118 ].
Newer HER2-targeted agents
To overcome intrinsic and acquired resistance to trastuzumab, various clinical trials have explored newer agents and combinations. The following innovative HER2-targeted agents for advanced metastatic GC are currently under investigation (Table 4 ): monoclonal antibodies (mAbs) (e.g., margetuximab), bispecific antibodies (BsAbs) (e.g., ZW25, KN026), antibody–drug conjugates (ADCs) (e.g., T-DXd, Disitamab vedotin, ARX788), tyrosine kinase inhibitors (TKIs) (e.g., tucatinib), and other novel therapeutic approaches.
Monoclonal antibodies
Margetuximab
Margetuximab is an Fc-engineered anti-HER2 mAb that targets the same epitope as trastuzumab but with a higher affinity for single-nucleotide polymorphisms of the activating Fc receptor (CD16A) [ 119 , 120 ]. Margetuximab can recruit CD16A-expressing natural killer cells, macrophages and monocytes and further promote antibody-dependent cell-mediated cytotoxicity (ADCC) [ 119 ]. The first phase I study of margetuximab in humans illustrated that margetuximab was well-tolerated with promising efficacy in relapsed HER2-overexpressing carcinoma [ 121 ]. Later in the phase Ib/II CP-MGAH22-05 study, patients with previously treated HER2-positive GC responded effectively to a chemotherapy-free treatment consisting of margetuximab plus pembrolizumab. Patients with HER2 IHC3 + and PD-L1 positive (CPS ≥ 1, by IHC) had an ORR of 44% and a DCR of 72% [ 122 ]. More recently, the phase II/III MAHOGANY trial has reported the efficacy of margetuximab plus anti-PD-1 antibody retifanlimab (Cohort A) for the first-line treatment of patients with G/GEJ adenocarcinoma, with an ORR and a DCR of 53% and 73% [ 123 ]. The ORR reported in this trial was superior to the ORR observed with other history chemotherapy-free treatments; nonetheless, given that chemotherapy-based regimens remain the predominant treatment for GC, the MAHOGANY trial has been halted for commercial reasons.
Bispecific antibodies (BsAbs)
Zanidatamab (ZW25)
Zanidatamab (ZW25) is a novel HER2-targeted bispecific antibody that binds to HER2 extracellular domain (ECD) II and IV. According to a phase I study, ZW25 was well tolerated with durable response in heavily pretreated GEA patients (including prior HER2-targeted therapy) [ 86 ]. Later in a phase II trial involving patients with advanced/metastatic HER2-positive GEA, zanidatamab plus chemotherapy (CAPOX or FP) showed a confirmed ORR of 75%, mDOR of 16.4 months and mPFS of 12.0 months in the first-line setting [ 124 ]. Based on these findings, a global phase III study (HERIZON-GEA-01) has been designed to assess the efficacy and safety profiles of zanidatamab plus chemotherapy with or without tislelizumab versus standard of care (trastuzumab plus chemotherapy) for patients with metastatic HER2-positive GEAs in first-line settings [ 125 ].
KN026 mimics the dual effects of trastuzumab and pertuzumab by simultaneously binding to HER2 ECD II and IV [ 126 ]. In a phase II clinical study, KN026 showed favorable results in patients with HER2-overexpressing G/GEJ adenocarcinoma (IHC3 + or IHC 2 + ISH +) with an ORR of 56% [ 127 ]. The ongoing phase II/III trial (KN026-001) is planned to evaluate the survival benefit of KN026 plus chemotherapy in patients with HER2-positive unresectable or advanced G/GEJ adenocarcinoma upon progression after trastuzumab-containing treatment (NCT05427383). Most recently, the preliminary data presented at ESMO 2022 illustrated that KN026 plus KN046, a recombinant humanized PD-L1/CTLA-4 bispecific antibody, had remarkable efficacy and tolerable safety in HER2-positive G/GEJ patients without prior systemic treatment [ 128 ]. In this phase II study, the ORR was 77.8%, and the DCR was 92.6%, indicating the need for a future randomized clinical trial to confirm the efficacy of KN026 plus KN046 treatment versus standard of care.
Other BsAbs
PRS-343 is a BsAb that targets HER2 and the costimulatory immunoreceptor 4-1BB on immune cells. In patients with advanced HER2-positive solid tumors, including GC, PRS-343 showed anticancer efficacy both alone and in combination with the anti-PD-L1 antibody atezolizumab in a phase I clinical study [ 129 ]. A phase II study (NCT05190445) is ongoing to investigate the efficacy of PRS-343 in combination with ramucirumab and paclitaxel in patients who have already received treatment for HER2-high (IHC 3+ or IHC 2+ with HER2/neu gene amplification) G/GEJ adenocarcinoma and in combination with tucatinib in HER2-low (IHC 1+ or IHC 2+ without HER2/neu gene amplification) G/GEJ adenocarcinoma.
Antibody–drug conjugates (ADCs)
Trastuzumab deruxtecan (T-DXd)
Trastuzumab deruxtecan (T-DXd) is an antibody–drug conjugate (ADC) composed of an anti-HER2 antibody connected to a cytotoxic topoisomerase I inhibitor via a cleavable tetrapeptide-based linker [ 130 ]. Different from T-DM1, T-DXd has a bystander effect on nearby cells, including those not expressing HER2, thus greatly enhancing the antitumor effect [ 131 ]. This action method is inspiring, particularly for advanced GC patients with diverse intratumoral HER2 expression. In the Asia DESTINY-Gastric01 trial, T-DXd significantly improved overall survival in patients with HER2 + advanced GC compared with chemotherapy in the later-line settings [ 132 ]. Interestingly, the efficacy and safety of T-DXd were also evaluated in exploratory cohorts of patients with HER2-low G/GEJ cancers in the DESTINY-Gastric01 trial (cohort 1, IHC 2 + /ISH–; cohort 2, IHC 1 +). The confirmed ORR was 26.3% in Cohort 1 and 9.5% in Cohort 2. The median OS was 7.8 months in cohort 1 and 8.5 months in cohort 2[ 133 ]. These results provide initial evidence that T-DXd has clinical benefits in patients with heavily pretreated HER2-low G/GEJ cancers.
Similarly, T-Dxd in the DESTINY-Gastric02 trial also achieved encouraging results in 2L western GC patients with a cORR of 41.8% and a median PFS of 5.6 months [ 134 ]. Other trials, such as phase III 2L DESTINY-Gastric04 and phase III 1L DESTINY-Gastric03, are also in progress (NCT04379596, NCT04704934).
Disitamab vedotin (RC48)
Disitamab vedotin (RC48) is a novel HER2-ADC drug independently developed in China, which is composed of three parts: anti-HER2 extracellular domain antibody, MC-Val-Cit-PAB linker, and cytotoxin monomethyl auristatin E (MMAE) [ 135 ]. This novel antibody has a stronger affinity to HER2 than the standard of care. Unlike T-DM1, disitamab vedotin has a bypass-killing effect on nearby tumor cells regardless of HER2 status, which could help overcome spatial heterogeneity and enhance anti-tumor effects. RC48 was well tolerated and showed promising antitumor activity in patients with HER2-positive advanced GC in a phase I trial [ 136 ]. The phase II RC48-C008 trial revealed a significant benefit of RC48 with HER2-overexpressing GC patients who had undergone at least two prior lines of therapy, in which the ORR was 24.8%, mPFS was 4.1 months and mOS was 7.9 months [ 137 ]. Of note, the ORR of RC48 in patients with HER2 IHC2 + /FISH- was 16.7%, slightly lower than in HER2-positive patients. These findings indicated that RC48 exerted considerable anti-tumor effectiveness and tolerable safety in patients with HER2-positive GC, as well as in those with HER2 low expression GC. In June 2021, disitamab vedotin was approved in China for the treatment of patients with HER2-overexpressing advanced or metastatic G/GEJ adenocarcinoma who received at least two systemic chemotherapy regimens. The ongoing phase III RC48-C007 (NCT04714190) trial aims to evaluate the efficacy and safety of RC48 as a third-line treatment and beyond in patients with advanced HER2-positive GC.
ARX788 is another investigational anti-HER2 antibody–drug conjugate consisting of HER2-targeted monoclonal antibody (mAb) coupled with a highly effective tubulin inhibitor (AS269). ARX788 was well tolerated and had a promising anti-tumor effect in HER2-positive GC patients previously treated with trastuzumab-based regimens in a phase I multicenter dosage expansion trial [ 138 ]. The ORR was confirmed to be 37.9%, and the DCR was 55.2%. With a median follow-up period of 10 months, the mPFS and OS were 4.1 and 10.7 months, respectively. On March 18, 2021, the FDA granted ARX788 as an orphan drug for treating HER2-positive GC. A randomized controlled, multicenter, open-label phase II/III study is underway to assess the efficacy of ARX788 as second-line treatment for HER2-positive advanced G/GEJ adenocarcinoma (Chinadrugtrials.org.cn: CTR20211583).
Tyrosine kinase inhibitors
Tucatinib, a highly selective HER2-directed tyrosine kinase inhibitor (TKI), was approved by FDA for HER2-positive metastatic breast cancer in 2020 and is under exploration in GC. In preclinical studies, tucatinib plus trastuzumab demonstrated superior activity compared to a single agent in GEC xenograft models [ 139 ]. Recently, the phase II/III MOUNTAINEER-02 (NCT04499924) was initiated to evaluate the efficacy of tucatinib, trastuzumab combined with ramucirumab, and paclitaxel in previously treated HER2 + advanced G/GEJ adenocarcinoma [ 140 ].
Other novel therapeutic approaches are being under investigation, including anti-HER2 CAR-T-cell therapy (NCT04511871, NCT04650451), CAR-natural killer cell (NK) therapy [ 141 ], and CAR-macrophage (CAR-M) therapy (NCT04660929), B-cell and monocyte-based immunotherapeutic vaccines (BVAC-B), BAY2701439 and CAM-H2 targeted HER2 radiotherapy (NCT04147819, NCT04467515). These widespread attempts at HER2-targeted CAR cell therapy in solid tumors may hopefully lead to the development of new drug candidates in patients with HER2-positive GC.
Antiangiogenic therapy
Blocking angiogenesis is a key strategy in GC therapy, including anti-VEGF monoclonal antibodies, VEGF-binding proteins, and VEGF receptor TKIs (Table 5 ) [ 142 ]. Ramucirumab, a typical antiangiogenic monoclonal antibody, targets VEGFR-2 and is approved by the FDA for treating advanced GC [ 143 ]. In the second-line REGARD trial, ramucirumab demonstrated significant improvement in patient OS and PFS versus best supportive care in metastatic GC [ 144 ]. In the RAINBOW trial, when coupled with paclitaxel, ramucirumab significantly prolonged overall survival compared to paclitaxel alone [ 145 ]. Similarly, results from RAINBOW-Asia bridging study also supported the application of ramucirumab plus paclitaxel as second-line therapy in a predominantly Chinese population with advanced gastric or GEJ adenocarcinoma [ 146 ]. However, neither ramucirumab nor bevacizumab brought extra survival benefits when added to platinum or fluoropyrimidine chemotherapy in GC patients in the first-line settings [ 147 , 148 ].
Regorafenib is an oral multi-kinase inhibitor targeting angiogenic, stromal and oncogenic receptor tyrosine kinases (RTK). Results from a phase III trial (INTEGRATE IIa) presented at ASCO GI 2023 demonstrated that regorafenib significantly improved OS (4.5 months vs. 4.0 months; HR = 0.52; P = 0.011) in patients with advanced gastro-oesophageal cancer (AGOC) in later-line settings [ 149 ]. Meanwhile, other studies exploring the efficacy of anti-VEGF and anti-PD1 combination in GC populations are also under investigation. The combination of regorafenib and nivolumab had a manageable safety profile and effective antitumor activity in a phase I trial for the GC subgroup [ 150 ]. INTEGRATE IIb ((NCT0487936)), an international randomized phase 3 trial, is ongoing to compare regorafenib plus nivolumab to standard chemotherapy in pre-treated patients with AGOC. Besides, lenvatinib plus pembrolizumab showed promising anti-tumor activity with an ORR of 69% in the first-line and second-line treatment of advanced GC [ 151 ].
Apatinib is a small molecule VEGFR inhibitor with China Food and Drug Administration (CFDA) approval for the treatment of advanced or metastatic chemotherapy-refractory GC. Apatinib improved median PFS and OS versus placebo in Chinese patients with advanced gastric or gastroesophageal junction adenocarcinoma in the third line and beyond[ 152 ]. Most of the patients in this trial did not receive prior antiangiogenic therapies since they were not standard treatments in China at that time, so clinical evidence is still lacking for the use of apatinib in patients who previously received ramucirumab. Unfortunately, no significant improvements were observed in overall survival (OS) in western populations in the phase III ANGEL clinical trial [ 153 ].
Fruquintinib is a highly selective VEGFR family kinase inhibitor that targets VEGFR1, 2 and 3 and is independently developed in China. Fruquintinib was approved in China by the NMPA in September 2018 and commercially launched in late November 2018 as a third-line treatment for patients with metastatic colorectal cancer. In a phase Ib/II study, adding fruquintinib to paclitaxel as second-line treatment for mGC patients at recommended phase 2 dose (RP2D) showed an mPFS of 4 months and mOS of 8.5 months. In the 4 mg dose cohort of 27 patients with evaluable tumor response, the ORR was 25.9% and the DCR was 66.7%[ 154 ]. A randomized phase III FRUTIGA study has investigated fruquintinib plus paclitaxel versus paclitaxel alone in patients with advanced gastric or gastroesophageal junction (GEJ) adenocarcinoma who had progressed after first-line standard chemotherapy (NCT03223376). Initial results from FRUTIGA showed that fruquintinib combined with paclitaxel showed significant improvements in PFS, ORR and DCR. Full detailed results are still being analyzed and will be revealed soon.
Other biomarker-targeted therapy
Novel diagnostic techniques have contributed to characterizing the genetic profile of GC and identifying new potential molecular targets. Recently, researchers have looked into Claudin-18.2-targeted therapy, fibroblast growth receptor (FGFR) pathway inhibitors, and EGFR inhibitors as effective targeted therapies to treat advanced GC (Table 5 ). Although emerging innovative drugs have made remarkable progress in GC treatments, extensive clinical explorations are needed to advance precision medicine.
CLAUDIN 18.2-targeted therapy
Claudin 18.2 (CLDN18.2), a component of intercellular junctions [ 155 ], is exclusively detected in gastric mucosa and absent from other healthy tissues. Upon malignant transformation, CLDN18.2 expression can be retained in various tumor tissues, including G/GEJ cancer and especially diffuse-type GC [ 156 ]. The prevalence of CLDN18.2 overexpression in GC varies wildly among studies ranging from 14.1% to 72% [ 157 , 158 , 159 ].
Zolbetuximab is a chimeric IgG1 monoclonal antibody that binds to CLDN18.2 and induces antibody-dependent and complement-dependent cytotoxicity [ 160 ]. To date, zolbetuximab has shown great potential to become a valuable target in GC. In the phase II MONO study, single-agent zolbetuximab achieved an ORR of 9% and a disease control rate of 23% in 43 patients with previously treated oesophageal or G/GEJ cancers [ 161 ]. A randomized phase II study (FAST) indicated that zolbetuximab plus first-line chemotherapy significantly improved PFS and OS in patients with CLDN18.2-positive G/GEJ cancer [ 159 ]. Subgroup analysis indicated a correlation between moderate-to-strong CLDN18.2 expression and a better overall survival rate. In the phase III SPOTLIGHT trial, zolbetuximab plus mFOLFOX6 significantly improved mPFS (10.61 vs 8.67 months, HR 0.751, P = 0.0066) and mOS (18.23 vs 15.54 months, HR 0.750, P = 0.0053) in patients with CLDN18.2-positive and HER-2-negative advanced G/GEJ cancer[ 162 ].
GLOW (NCT03653507) is another phase III trial investigating zolbetuximab plus CAPOX as first-line treatment in patients with CLDN18.2-positive, HER2-negative, locally advanced unresectable or metastatic gastric or GEJ cancer. In this study, zolbetuximab plus CAPOX showed a significant improvement in mPFS (8.21 vs 6.80 months, HR 0.687, P = 0.0007) and mOS (14.39 vs 12.16 months, HR 0.771, P = 0.0118) compared to placebo plus CAPOX[ 163 ]. Additionally, zolbetuximab is also being studied in combination with immunotherapy in patients with CLDN18.2-positive advanced gastric or GEJ cancer in the ILUSTRO study (NCT03505320).
Another promising therapeutic approach targeting CLDN18.2 employs CLDN18.2-specific chimeric antigen receptor (CAR) T cells. CLDN18.2-specific CAR T cells achieved partial or complete tumor regression in CLDN18.2-positive PDX models [ 164 ]. A phase I study of CLDN18.2-specific CAR T cells in gastrointestinal cancers conducted by Prof. Shen Lin's team demonstrated that in GC patients, the ORR and DCR were 57.1% and 75.0%, respectively, and the 6-month overall survival rate was 81.2% [ 165 ]. Claudin 18.2 served as a new target for the later-line treatment of GC, with considerable ORR improvement achieved in Claudin 18.2 CAR-T therapy, which has become a hallmark event for cellular immunotherapy in solid tumors. Currently, several new drugs focusing on Claudin 18.2, such as Claudin 18.2 bispecific antibodies (Claudin 18.2/CD3, Claudin 18.2/PD-L1) and ADC analogs, are being developed. Although these drugs have not been approved for clinical applications, some of them showed promising preclinical data and are being widely studied in different clinical trials. Since Claudin 18.2 is also expressed on the normal gastric mucosal epithelial surface, the risk of adverse reactions and whether ADC drugs may aggravate normal mucosal damage should also be a concern.
FGFR-targeted therapy
FGFR1 mutations, FGFR2 amplifications, and FGFR3 rearrangements are the most common FGFR alterations in GC [ 166 ]. Different types of FGFR targeting agents were explored or developed in GC, including multikinase inhibitors, pan-FGFR inhibitors, FGFR1-3 inhibitors, selective FGFR inhibitors and ADC. Nevertheless, most multikinase inhibitor studies were preclinical or single case reports in GC without robust clinical evidence [ 167 ]. Futibatinib, an irreversible and highly selective FGFR1–4 inhibitor that permanently disables FGFR2, has been tested in a phase II trial involving patients with advanced-stage solid tumors harboring FGFR alterations, including those with FGFR2-amplified G/GEJ cancers [ 168 ]. Although the ORR was reported to be 22.2% in the GC cohort [ 169 ], more data are needed to support the efficacy of multiple FGFR inhibitors in different FGFR gene alterations in GC.
Currently, bemarituzumab has shown some promising results in the treatment of mGC [ 170 ]. It is a first-in-class afucosylated monoclonal antibody against the FGFR2b splice variant frequently overexpressed in FGFR2- amplified G/GEJ cancers. In a phase I trial, 17.9% of patients with FGFR2 amplifications had a confirmed response to bemarituzumab [ 171 ]. Based on the safety and activity profile of bemarituzumab monotherapy in GC, the phase II FIGHT trial was designed to evaluate the efficacy of bemarituzumab plus mFOLFOX6 regimen in previously untreated, FGFR2b-overexpressing advanced-stage G/GEJ cancers [ 172 ]. The trial showed a 2-month improvement in PFS, and the OS was not reached (NR) in the experimental arm (bemarituzumab + mFOLFOX6). However, the experimental arm had a higher incidence of adverse events than the control chemotherapy arm, particularly in regard to ocular toxicity.
EGFR-targeted therapy
Approximately 5–10% of patients with G/GEJ cancers have EGFR amplifications or EGFR overexpression, both of which are associated with poor prognosis [ 173 ]. Previous large randomized clinical trials have failed to demonstrate any significant survival benefit with EGFR-targeted agents [ 92 , 174 ], perhaps because most of the studies were performed in unselected patient populations regardless of EGFR status. Besides, biomarker analysis of the EXPAND and COG trials suggests activity in patients with tumors expressing high levels of EGFR, thus supporting the significance of patient selection for future trials [ 175 , 176 ]. In a prospective cohort, patients with metastatic gastroesophageal adenocarcinoma were screened for EGFR amplification and subsequently treated with anti-EGFR therapy (cetuximab). The ORR was 58% (4 of 7 patients), and the DCR was 100% (7 of 7 patients), implying that EGFR inhibition should be further studied in selected patients [ 177 ]. Many of the ongoing EGFR inhibitor studies should test EGFR alterations in the GC patients prior to enrollment to overcome resistance to EGFR-targeted therapies.
MET/HGF pathway inhibitors
c-Mesenchymal-Epithelial Transition (c-MET) is a tyrosine kinase receptor from MET families, and hepatocyte growth factor (HGF) is the common ligand to c-MET [ 178 ]. MET/HGF pathway activation is associated with tumor invasiveness and poor disease prognosis. The anti-MET monoclonal antibody, onartuzumab, has been studied in a phase III trial of onartuzumab plus mFOLFOX6 vs placebo plus mFOLFOX6 in patients with metastatic HER2-negative G/GEJ cancers. However, the addition of onartuzumab to mFOLFOX6 did not improve clinical outcomes in the ITT population or in the MET-positive population [ 179 ]. Rilotumumab is a humanized monoclonal antibody targeting HGF. Two phase III trials (RILOMET-1 and RILOMET-2) investigated rilotumumab plus chemotherapy in advanced MET-positive G/GEJ cancers. Unfortunately, both studies were terminated due to increased number of deaths in the rilotumumab group[ 180 , 181 ]. Additionally, several selective/non-selective c-MET TKIs, such as tinvatinib, AMG 337 and foretinib, have also been tested in MET-positive GC, but no significant benefit was seen in clinical trials[ 182 , 182 , 184 ].
Challenges and future perspectives
Even though substantial advances have been made in the treatment of GC, further research and development are still necessary. Improving early detection, reducing recurrence and optimizing treatment strategies are the primary challenges and prospects for GC management. To increase GC early detection and promote patients’ overall survival, endoscopic screening programs should be implemented in high-risk regions, and more precise early detection technologies are of great value. In a previous study, we demonstrated an artificial intelligence (AI) diagnostic platform, GRAIDS, to detect upper gastrointestinal cancers using real-world endoscopic imaging data from six Chinese hospitals with varying experience in the endoscopic diagnosis of upper gastrointestinal cancer [ 185 ]. GRAIDS provided both real-time and retrospective assistance for enhancing the effectiveness of upper gastrointestinal cancer screening and diagnosis, with high diagnostic accuracy and sensitivity in detecting upper gastrointestinal cancers. In the near future, the AI system will help many physicians in community-based hospitals identify upper gastrointestinal cancers more efficiently and accurately [ 186 ].
In addition, recurrence of GC remains common despite the multimodality treatment, so many studies in progress aim to identify individuals at risk of recurrence after treatment. Circulating tumor DNA (ctDNA) can be detected in the circulation of cancer patients and has the potential to predict minimal residual disease [ 187 ]. Liquid biopsies can detect a broader spectrum of abnormalities in a heterogeneous tumor compared to conventional tissue biopsies. According to a study investigating perioperative therapies in patients in the CRITICS trial with resectable GC, the presence of ctDNA could predict recurrence when analyzed within nine weeks after preoperative treatment and after surgery in patients eligible for multimodal treatment [ 187 ]. These findings highlight the significance of ctDNA as a biomarker for predicting patient outcomes following perioperative cancer treatment and surgical resection in patients with GC. In another 1630-patient cohort of ctDNA results, genomic alterations were correlated with clinicopathologic characteristics and outcomes and provided prognostic and predictive information [ 188 ]. As for advanced GC, ctDNA also serves as a potential biomarker of immunotherapy response, and its potential role in predicting irAEs is worth further investigation [ 189 ]. Further research aimed at prospectively collecting ctDNA is needed to confirm these findings. The existence of persistent ctDNA following curative-intent treatment of GC may indicate minimal residual disease, and trials are underway to determine whether additional adjuvant therapy can result in the clearance of ctDNA.
Intratumoral, intrapatient, and interpatient heterogeneity in GC is the major barrier to drug development for systemic therapies. Most GC patients are not susceptible to immune checkpoint inhibitor monotherapies. Thus, one of the major challenges in systemic treatments for GC is overcoming resistance to ICI therapy. One strategy is to develop novel ICIs with better efficacy. Recently, many novel immune checkpoint modulators have been widely investigated, including LAG-3, VISTA, TIM-3, TIGIT, CD38, CD39, and CD73[ 190 ]. Another key strategy is combining ICI and other therapies, such as other ICI, targeted therapies, other immune-modulating agents, chemotherapy (as discussed above), and radiotherapy [ 191 ]. As mentioned above, in the CheckMate-649 study, the combination of anti-PD-1 and anti-CTLA-4 agents (nivolumab plus ipilimumab) failed to improve treatment outcomes compared to traditional chemotherapy [ 57 ]. In the EPOC1706 study, lenvatinib, an anti-angiogenic multiple receptor tyrosine kinase inhibitor, combined with pembrolizumab showed an exciting activity with an ORR of 69% in the first-line and second-line treatment of advanced GC[ 151 ]. ICI combined with other anti-immunosuppressive factor agents, such as anti-transforming growth factor-β (TGF-β), is also being investigated in clinical trials (NCT04856774). To fully understand the mechanism of resistance to immunotherapy, factors such as epigenetics, metabolism, immune suppression, and microbiota must be considered. Therefore, the development of combined therapies should be based on understanding the underlying mechanisms of immune modulation and resistance, rather than simply combining available therapies in a haphazard manner.
Rapid developments are ongoing in the clinical use of ADCs and are now considered one of the current hot spots for antitumor drug development. In particular, ADCs have emerged as a new era of targeted therapy in the field of GC treatment. The latest generation of ADCs has expanded the treatment population to include novel targets and demonstrated superior clinical outcomes compared to traditional chemotherapy drugs. Nevertheless, certain aspects of ADCs remain to be addressed. Firstly, it is necessary to explore ways to advance ADCs as first-line therapy to benefit a larger number of patients. Secondly, to make better use of medical resources, a more differentiated target layout needs to be established, moving beyond the focus on distinct targets such as HER2. To address these challenges, optimization of the toxin, linker and toxicity of ADCs is essential, along with the development of ADC-combination therapies to improve efficacy. We anticipate the discovery of more potential ADC drugs and expect a breakthrough in first-line treatment.
Currently, many clinical trials have complex treatment regimens, including mono-immunotherapy, double-checkpoint inhibitors, anti-angiogenic drugs, and biomarker-directed therapies [ 190 , 192 ]. However, the challenge of determining the optimal treatment strategy and the appropriate timing of molecular biomarker screening has yet to be resolved. We expect that extensive translational research, preclinical investigations, and multi-omics-based clinical trials will lead to breakthroughs in the diagnosis and treatment of GC. Therefore, we eagerly anticipate future studies that have the potential to improve clinical practice in the coming years.
Availability of data and materials
Not applicable.
Abbreviations
Antibody–drug conjugates
Antibody-dependent cell-mediated cytotoxicity
Advanced gastro-oesophageal cancer
Artificial intelligence
American Society of Clinical Oncology
Bispecific antibodies
Best supportive care
Capecitabine and oxaliplatin
Chimeric antigen receptor
Cisplatin and fluorouracil
China Food and Drug Administration
Confidence interval
Claudin18.2
Combined positive score
Chemoradiotherapy
Chinese Society for Clinical Oncology
Circulating tumor DNA
Cytotoxic T lymphocyte antigen-4
Disease control rate
Disease-free survival
Mismatch-repair deficiency
Duration of response
Docetaxel, oxaliplatin, and S-1
Epstein-Barr virus
Epstein-Barr virus-associated gastric cancer
Extracellular domain
Epirubicin, cisplatin, and fluorouracil
Event-free survival
Epidermal growth factor receptor
Epirubicin and oxaliplatin
Erythroblastic leukemia viral oncogene homolog
Extracellular regulated protein kinase
European Society for Medical Oncology
Food and Drug Administration
Fibroblast growth factor receptor
Fluorescent in situ hybridization
Fluorouracil, leucovorin, oxaliplatin, and docetaxel
Fluorouracil, leucovorin, and oxaliplatin
Fluorouracil and cisplatin
- Gastric cancer
Gastroesophageal junction adenocarcinoma
Gastroesophageal junction
Human epidermal growth factor receptor 2
Hazard ratio
Immune checkpoint inhibitor
Immunohistochemistry
Kirsten rats sarcomaviral oncogene homolog
Lymphocyte-activation gene 3
Mitogen-activated protein kinase
Mesenchymal epithelial transition
Cytotoxin monomethyl auristatin E
Microsatellite instability
Mammalian target of rapamycin
National Comprehensive Cancer Network
Not evaluable
Natural killer
Objective response rate
Overall survival
Pathological complete response
Programmed cell death 1
Programmed cell death ligand 1
Progression-free survival
Phosphatidylinositol-3-kinase
Phosphatase and tensin homolog
Relapse-free survival
Recommended phase 2 dose
Receptor tyrosine kinase
S-1 and oxaliplatin
S-1, oxaliplatin and radiotherapy
S-1 and cisplatin
Tumor area positivity
The Cancer Genome Atlas
Trastuzumab emtansine
Trastuzumab deruxtecan
Transforming growth factor-β
T cell immunoreceptor with Ig and ITIM domain
T cell immunoglobulin and mucin domain 3
Tumor mutational burden
Vascular endothelial growth factor
V-domain Ig suppressor of T cell activation
Capecitabine and cisplatin
Siegel RL, et al. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33.
Article PubMed Google Scholar
Sung H, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Tan P, Yeoh KG. Genetics and molecular pathogenesis of gastric adenocarcinoma. Gastroenterology. 2015;149(5):1153–62.
Article CAS PubMed Google Scholar
Tramacere I, et al. A meta-analysis on alcohol drinking and gastric cancer risk. Ann Oncol. 2012;23(1):28–36.
Lordick F, et al. Gastric cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33(10):1005–20.
Lu L, et al. A global assessment of recent trends in gastrointestinal cancer and lifestyle-associated risk factors. Cancer Commun (Lond). 2021;41(11):1137–51.
Pennathur A, et al. Oesophageal carcinoma. Lancet. 2013;381(9864):400–12.
Qiu H, Cao S, Xu R. Cancer incidence, mortality, and burden in China: a time-trend analysis and comparison with the United States and United Kingdom based on the global epidemiological data released in 2020. Cancer Commun (Lond). 2021;41(10):1037–48.
Wagner AD, et al. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev. 2017;8:CD004064.
PubMed Google Scholar
Korfer J, Lordick F, Hacker UT. Molecular targets for gastric cancer treatment and future perspectives from a clinical and translational point of view. Cancers (Basel), 2021;13(20).
Cancer Genome Atlas Research N. Comprehensive molecular characterization of gastric adenocarcinoma. Nature, 2014;513(7517): 202–9.
Salem ME, et al. Comparative molecular analyses of esophageal squamous cell carcinoma, esophageal adenocarcinoma, and gastric adenocarcinoma. Oncologist. 2018;23(11):1319–27.
Article CAS PubMed PubMed Central Google Scholar
Wang J, et al. Large-scale analysis of KMT2 mutations defines a distinctive molecular subset with treatment implication in gastric cancer. Oncogene. 2021;40(30):4894–905.
Bang YJ, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376(9742):687–97.
Chao J, et al. Assessment of pembrolizumab therapy for the treatment of microsatellite instability-high gastric or gastroesophageal junction cancer among patients in the KEYNOTE-059, KEYNOTE-061, and KEYNOTE-062 Clinical Trials. JAMA Oncol. 2021;7(6):895–902.
Article PubMed PubMed Central Google Scholar
Nakamura Y, et al. Biomarker-targeted therapies for advanced-stage gastric and gastro-oesophageal junction cancers: an emerging paradigm. Nat Rev Clin Oncol. 2021;18(8):473–87.
Wang FH, et al. The Chinese Society of Clinical Oncology (CSCO): Clinical guidelines for the diagnosis and treatment of gastric cancer, 2021. Cancer Commun (Lond). 2021;41(8):747–95.
Ajani JA, et al. Gastric cancer, version 2, 2022, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2022;20(2):167–92.
Article CAS Google Scholar
Cunningham D, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355(1):11–20.
Ychou M, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011;29(13):1715–21.
Al-Batran SE, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet. 2019;393(10184):1948–57.
Kang YK, et al. PRODIGY: a phase III study of neoadjuvant docetaxel, oxaliplatin, and S-1 plus surgery and adjuvant S-1 versus surgery and adjuvant S-1 for resectable advanced gastric cancer. J Clin Oncol. 2021;39(26):2903–13.
Yoshida K, et al. Addition of docetaxel to oral fluoropyrimidine improves efficacy in patients with stage III gastric cancer: interim analysis of JACCRO GC-07, a randomized controlled trial. J Clin Oncol. 2019;37(15):1296–304.
Zhang X, et al. Perioperative or postoperative adjuvant oxaliplatin with S-1 versus adjuvant oxaliplatin with capecitabine in patients with locally advanced gastric or gastro-oesophageal junction adenocarcinoma undergoing D2 gastrectomy (RESOLVE): an open-label, superiority and non-inferiority, phase 3 randomised controlled trial. Lancet Oncol. 2021;22(8):1081–92.
Japanese Gastric Cancer, A.Japanese Gastric Cancer Treatment Guidelines 2021 (6th edition). Gastric Cancer, 2023;26(1): 1–25.
Bang YJ, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012;379(9813):315–21.
Noh SH, et al. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15(12):1389–96.
Sasako M, et al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011;29(33):4387–93.
Pietrantonio F, et al. Individual patient data meta-analysis of the value of microsatellite instability as a biomarker in gastric cancer. J Clin Oncol. 2019;37(35):3392–400.
Macdonald JS, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345(10):725–30.
Lee J, et al. Phase III trial comparing capecitabine plus cisplatin versus capecitabine plus cisplatin with concurrent capecitabine radiotherapy in completely resected gastric cancer with D2 lymph node dissection: the ARTIST trial. J Clin Oncol. 2012;30(3):268–73.
Park SH, et al. A randomized phase III trial comparing adjuvant single-agent S1, S-1 with oxaliplatin, and postoperative chemoradiation with S-1 and oxaliplatin in patients with node-positive gastric cancer after D2 resection: the ARTIST 2 trial. Ann Oncol. 2021;32(3):368–74.
Hofheinz RD, et al. Trastuzumab in combination with 5-fluorouracil, leucovorin, oxaliplatin and docetaxel as perioperative treatment for patients with human epidermal growth factor receptor 2-positive locally advanced esophagogastric adenocarcinoma: A phase II trial of the Arbeitsgemeinschaft Internistische Onkologie Gastric Cancer Study Group. Int J Cancer. 2021;149(6):1322–31.
Hofheinz RD, et al. Perioperative trastuzumab and pertuzumab in combination with FLOT versus FLOT alone for HER2-positive resectable esophagogastric adenocarcinoma: final results of the PETRARCA multicenter randomized phase II trial of the AIO. J Clin Oncol. 2020;38(15_suppl):4502–4502.
Article Google Scholar
Rivera F, et al. Perioperative trastuzumab, capecitabine and oxaliplatin in patients with HER2-positive resectable gastric or gastro-oesophageal junction adenocarcinoma: NEOHX phase II trial. Eur J Cancer. 2021;145:158–67.
Wagner AD, et al. EORTC-1203-GITCG - the “INNOVATION”-trial: Effect of chemotherapy alone versus chemotherapy plus trastuzumab, versus chemotherapy plus trastuzumab plus pertuzumab, in the perioperative treatment of HER2 positive, gastric and gastroesophageal junction adenocarcinoma on pathologic response rate: a randomized phase II-intergroup trial of the EORTC-Gastrointestinal Tract Cancer Group, Korean Cancer Study Group and Dutch Upper GI-Cancer group. BMC Cancer. 2019;19(1):494.
Cunningham D, et al. Peri-operative chemotherapy with or without bevacizumab in operable oesophagogastric adenocarcinoma (UK Medical Research Council ST03): primary analysis results of a multicentre, open-label, randomised phase 2–3 trial. Lancet Oncol. 2017;18(3):357–70.
Goetze TO, et al. Perioperative ramucirumab in combination with FLOT versus FLOT alone for resectable esophagogastric adenocarcinoma (RAMSES/FLOT7) with high rate of signet cell component: final results of the multicenter, randomized phase II/III trial of the German AIO and Italian GOIM. J Clin Oncol. 2022;40(16_suppl):4042–4042.
Al-Batran S-E, et al. Surgical and pathological outcome, and pathological regression, in patients receiving perioperative atezolizumab in combination with FLOT chemotherapy versus FLOT alone for resectable esophagogastric adenocarcinoma: Interim results from DANTE, a randomized, multicenter, phase IIb trial of the FLOT-AIO German Gastric Cancer Group and Swiss SAKK. J Clin Oncol. 2022;40(16_suppl):4003–4003.
Liu Y, et al. Camrelizumab combined with FLOFOX as neoadjuvant therapy for resectable locally advanced gastric and gastroesophageal junction adenocarcinoma: updated results of efficacy and safety. J Clin Oncol. 2021;39(15_suppl):4036.
Li H, et al. Phase II study of perioperative toripalimab in combination with FLOT in patients with locally advanced resectable gastric/gastroesophageal junction (GEJ) adenocarcinoma. J Clin Oncol. 2021;39(15_suppl):4050–4050.
Alcindor T, et al. Phase II trial of perioperative chemotherapy + avelumab in locally advanced gastroesophageal adenocarcinoma: preliminary results. J Clin Oncol. 2021;39(15_suppl):4046–4046.
Li S, et al. A prospective, phase II, single-arm study of neoadjuvant/conversion therapy with camrelizumab, apatinib, S-1 ± oxaliplatin for locally advanced cT4a/bN+ gastric cancer. J Clin Oncol. 2021;39(15_suppl):4061.
Wei J, et al. SHARED: Efficacy and safety of sintilimab in combination with concurrent chemoradiotherapy (cCRT) in patients with locally advanced gastric (G) or gastroesophageal junction (GEJ) adenocarcinoma. J Clin Oncol. 2021;39(15_suppl):4040–4040.
Bang YJ, et al. KEYNOTE-585: Phase III study of perioperative chemotherapy with or without pembrolizumab for gastric cancer. Future Oncol. 2019;15(9):943–52.
Janjigian YY, et al. MATTERHORN: efficacy and safety of neoadjuvant-adjuvant durvalumab and FLOT chemotherapy in resectable gastric and gastroesophageal junction cancer—a randomized, double-blind, placebo-controlled, phase 3 study. J Clin Oncol. 2021;39(15):TPS4151
Andre T, et al. neoadjuvant nivolumab plus ipilimumab and adjuvant nivolumab in localized deficient mismatch repair/microsatellite instability-high gastric or esophagogastric junction adenocarcinoma: the GERCOR NEONIPIGA Phase II Study. J Clin Oncol. 2023;41(2):255–65.
Pietrantonio F, et al. INFINITY: A multicentre, single-arm, multi-cohort, phase II trial of tremelimumab and durvalumab as neoadjuvant treatment of patients with microsatellite instability-high (MSI) resectable gastric or gastroesophageal junction adenocarcinoma (GAC/GEJAC). J Clin Oncol. 2023;41(4_suppl):358–358.
Xu R-H, et al. S-1 plus oxaliplatin versus S-1 plus cisplatin as first-line treatment for advanced diffuse-type or mixed-type gastric/gastroesophageal junction adenocarcinoma: a randomized, phase 3 trial. J Clin Oncol. 2019;37(15_suppl):4017–4017.
Hall PS, et al. efficacy of reduced-intensity chemotherapy with oxaliplatin and capecitabine on quality of life and cancer control among older and frail patients with advanced gastroesophageal cancer: the go2 phase 3 randomized clinical trial. JAMA Oncol. 2021;7(6):869–77.
Shitara K, et al. Nab-paclitaxel versus solvent-based paclitaxel in patients with previously treated advanced gastric cancer (ABSOLUTE): an open-label, randomised, non-inferiority, phase 3 trial. Lancet Gastroenterol Hepatol. 2017;2(4):277–87.
Shitara K, et al. Trifluridine/tipiracil versus placebo in patients with heavily pretreated metastatic gastric cancer (TAGS): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2018;19(11):1437–48.
Shitara K, et al. Efficacy and safety of pembrolizumab or pembrolizumab plus chemotherapy vs chemotherapy alone for patients with first-line, advanced gastric cancer: the KEYNOTE-062 phase 3 randomized clinical trial. JAMA Oncol. 2020;6(10):1571–80.
Wainberg ZA, et al. Pembrolizumab with or without chemotherapy versus chemotherapy alone for patients with PD-L1–positive advanced gastric or gastroesophageal junction adenocarcinoma: Update from the phase 3 KEYNOTE-062 trial. J Clin Oncol. 2022;40(4_suppl):243–243.
Rha SY, et al. VP1-2023: Pembrolizumab (pembro) plus chemotherapy (chemo) as first-line therapy for advanced HER2-negative gastric or gastroesophageal junction (G/GEJ) cancer: Phase III KEYNOTE-859 study. Ann Oncol. 2023;34(3):319–20.
Janjigian YY, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398(10294):27–40.
Janjigian YY, et al. LBA7 Nivolumab (NIVO) plus chemotherapy (Chemo) or ipilimumab (IPI) vs chemo as first-line (1L) treatment for advanced gastric cancer/gastroesophageal junction cancer/esophageal adenocarcinoma (GC/GEJC/EAC): CheckMate 649 study. Ann Oncol. 2021;32:S1329–30.
Kang YK, et al. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): a randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022;23(2):234–47.
Xu J, et al. LBA53 Sintilimab plus chemotherapy (chemo) versus chemo as first-line treatment for advanced gastric or gastroesophageal junction (G/GEJ) adenocarcinoma (ORIENT-16): first results of a randomized, double-blind, phase III study. Ann Oncol. 2021;32:S1331.
Xu R-h, et al. Tislelizumab plus chemotherapy versus placebo plus chemotherapy as first-line therapy in patients with locally advanced unresectable or metastatic gastric or gastroesophageal junction (G/GEJ) adenocarcinoma. J Clin Oncol 2020;38(4_suppl): TPS458
Moehler MH, et al. Rationale 305: Phase 3 study of tislelizumab plus chemotherapy vs placebo plus chemotherapy as first-line treatment (1L) of advanced gastric or gastroesophageal junction adenocarcinoma (GC/GEJC). J Clin Oncol. 2023;41(4):286–286.
Moehler M, et al. Phase III trial of avelumab maintenance after first-line induction chemotherapy versus continuation of chemotherapy in patients with gastric cancers: results from JAVELIN gastric 100. J Clin Oncol. 2021;39(9):966–77.
Fuchs CS, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. 2018;4(5): e180013.
Kang YK, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390(10111):2461–71.
Bang YJ, et al. Phase III, randomised trial of avelumab versus physician’s choice of chemotherapy as third-line treatment of patients with advanced gastric or gastro-oesophageal junction cancer: primary analysis of JAVELIN Gastric 300. Ann Oncol. 2018;29(10):2052–60.
Triulzi T, et al. HER2 signaling regulates the tumor immune microenvironment and trastuzumab efficacy. Oncoimmunology. 2019;8(1): e1512942.
Varadan V, et al. Immune signatures following single dose trastuzumab predict pathologic response to preoperativetrastuzumab and chemotherapy in HER2-positive early breast cancer. Clin Cancer Res. 2016;22(13):3249–59.
Chaganty BKR, et al. Trastuzumab upregulates PD-L1 as a potential mechanism of trastuzumab resistance through engagement of immune effector cells and stimulation of IFNgamma secretion. Cancer Lett. 2018;430:47–56.
Stagg J, et al. Anti-ErbB-2 mAb therapy requires type I and II interferons and synergizes with anti-PD-1 or anti-CD137 mAb therapy. Proc Natl Acad Sci U S A. 2011;108(17):7142–7.
Takahari D, et al. A phase Ib study of nivolumab plus trastuzumab with S-1/capecitabine plus oxaliplatin for HER2-positive advanced gastric cancer (Ni-HIGH study): safety evaluation. J Clin Oncol. 2020;38(15_suppl): 4525
Rha SY, et al. A multi-institutional phase Ib/II trial of first-line triplet regimen (Pembrolizumab, Trastuzumab, Chemotherapy) for HER2-positive advanced gastric and gastroesophageal junction cancer (PANTHERA Trial): Molecular profiling and clinical update. J Clin. Oncol.2021;39(3_suppl):218.
Janjigian YY, et al. First-line pembrolizumab and trastuzumab in HER2-positive oesophageal, gastric, or gastro-oesophageal junction cancer: an open-label, single-arm, phase 2 trial. Lancet Oncol. 2020;21(6):821–31.
Janjigian YY, et al. The KEYNOTE-811 trial of dual PD-1 and HER2 blockade in HER2-positive gastric cancer. Nature. 2021;600(7890):727–30.
Guan WL, et al. The impact of mismatch repair status on prognosis of patients with gastric cancer: a multicenter analysis. Front Oncol. 2021;11: 712760.
Vanderwalde A, et al. Microsatellite instability status determined by next-generation sequencing and compared with PD-L1 and tumor mutational burden in 11,348 patients. Cancer Med. 2018;7(3):746–56.
Pietrantonio F, et al. Predictive role of microsatellite instability for PD-1 blockade in patients with advanced gastric cancer: a meta-analysis of randomized clinical trials. ESMO Open. 2021;6(1): 100036.
Huang SC, et al. Subtraction of Epstein-Barr virus and microsatellite instability genotypes from the Lauren histotypes: combined molecular and histologic subtyping with clinicopathological and prognostic significance validated in a cohort of 1,248 cases. Int J Cancer. 2019;145(12):3218–30.
Qiu MZ, et al. Prospective observation: clinical utility of plasma Epstein-Barr virus DNA load in EBV-associated gastric carcinoma patients. Int J Cancer. 2020;146(1):272–80.
Derks S, et al. Abundant PD-L1 expression in Epstein-Barr Virus-infected gastric cancers. Oncotarget. 2016;7(22):32925–32.
Kim ST, et al. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat Med. 2018;24(9):1449–58.
Sun YT, et al. PD-1 antibody camrelizumab for Epstein-Barr virus-positive metastatic gastric cancer: a single-arm, open-label, phase 2 trial. Am J Cancer Res. 2021;11(10):5006–15.
CAS PubMed PubMed Central Google Scholar
Xie T, et al. Positive status of Epstein-Barr virus as a biomarker for gastric cancer immunotherapy: a prospective observational study. J Immunother. 2020;43(4):139–44.
Mishima S, et al. Clinicopathological and molecular features of responders to nivolumab for patients with advanced gastric cancer. J Immunother Cancer. 2019;7(1):24.
Shitara K, et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet. 2018;392(10142):123–33.
Wainberg ZA, et al. Efficacy of pembrolizumab monotherapy for advanced gastric/gastroesophageal junction cancer with programmed death ligand 1 combined positive score >/=10. Clin Cancer Res. 2021;27(7):1923–31.
Moehler MH, et al. First-line (1L) nivolumab (NIVO) plus chemotherapy (chemo) versus chemo in advanced gastric cancer/gastroesophageal junction cancer/esophageal adenocarcinoma (GC/GEJC/EAC): expanded efficacy and safety data from CheckMate 649. J Clin Oncol. 2021;39(15_suppl): 4002
Zhao JJ, et al. Low programmed death-ligand 1-expressing subgroup outcomes of first-line immune checkpoint inhibitors in gastric or esophageal adenocarcinoma. J Clin Oncol. 2022;40(4):392–402.
Wang F, et al. Safety, efficacy and tumor mutational burden as a biomarker of overall survival benefit in chemo-refractory gastric cancer treated with toripalimab, a PD-1 antibody in phase Ib/II clinical trial NCT02915432. Ann Oncol. 2019;30(9):1479–86.
Shitara K, et al. The association of tissue tumor mutational burden (tTMB) using the Foundation Medicine genomic platform with efficacy of pembrolizumab versus paclitaxel in patients (pts) with gastric cancer (GC) from KEYNOTE-061. J Clin Oncol. 2020;38(15_suppl): 4537
Marabelle A, et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020;21(10):1353–65.
Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2(2):127–37.
Abrahao-Machado LF, Scapulatempo-Neto C. HER2 testing in gastric cancer: an update. World J Gastroenterol. 2016;22(19):4619–25.
Van Cutsem E, et al. HER2 screening data from ToGA: targeting HER2 in gastric and gastroesophageal junction cancer. Gastric Cancer. 2015;18(3):476–84.
Qin S, et al. Treatment patterns and outcomes in chinese patients with gastric cancer by HER2 status: a noninterventional registry study (EVIDENCE). Oncologist. 2021;26(9):e1567–80.
Tabernero J, et al. Pertuzumab plus trastuzumab and chemotherapy for HER2-positive metastatic gastric or gastro-oesophageal junction cancer (JACOB): final analysis of a double-blind, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2018;19(10):1372–84.
Liu T, et al. Pertuzumab in combination with trastuzumab and chemotherapy for Chinese patients with HER2-positive metastatic gastric or gastroesophageal junction cancer: a subpopulation analysis of the JACOB trial. Cancer Commun (Lond). 2019;39(1):38.
Thuss-Patience PC, et al. Trastuzumab emtansine versus taxane use for previously treated HER2-positive locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma (GATSBY): an international randomised, open-label, adaptive, phase 2/3 study. Lancet Oncol. 2017;18(5):640–53.
Hecht JR, et al. Lapatinib in combination with capecitabine plus oxaliplatin in human epidermal growth factor receptor 2-positive advanced or metastatic gastric, esophageal, or gastroesophageal adenocarcinoma: TRIO-013/LOGiC–a randomized phase III trial. J Clin Oncol. 2016;34(5):443–51.
Satoh T, et al. Lapatinib plus paclitaxel versus paclitaxel alone in the second-line treatment of HER2-amplified advanced gastric cancer in Asian populations: TyTAN–a randomized, phase III study. J Clin Oncol. 2014;32(19):2039–49.
Makiyama A, et al. Randomized, phase II study of trastuzumab beyond progression in patients with HER2-positive advanced gastric or gastroesophageal junction cancer: WJOG7112G (T-ACT study). J Clin Oncol. 2020;38(17):1919–27.
Yagi S, et al. Clinical significance of intratumoral HER2 heterogeneity on trastuzumab efficacy using endoscopic biopsy specimens in patients with advanced HER2 positive gastric cancer. Gastric Cancer. 2019;22(3):518–25.
Nishida Y, et al. A novel gene-protein assay for evaluating HER2 status in gastric cancer: simultaneous analyses of HER2 protein overexpression and gene amplification reveal intratumoral heterogeneity. Gastric Cancer. 2015;18(3):458–66.
Lee HJ, et al. Clinicopathologic significance of the intratumoral heterogeneity of HER2 gene amplification in HER2-positive breast cancer patients treated with adjuvant trastuzumab. Am J Clin Pathol. 2015;144(4):570–8.
Kim KC, et al. Evaluation of HER2 protein expression in gastric carcinomas: comparative analysis of 1,414 cases of whole-tissue sections and 595 cases of tissue microarrays. Ann Surg Oncol. 2011;18(10):2833–40.
Haffner I, et al. HER2 expression, test deviations, and their impact on survival in metastatic gastric cancer: results from the prospective multicenter VARIANZ study. J Clin Oncol. 2021;39(13):1468–78.
Palle J, et al. Human epidermal growth factor receptor 2 (HER2) in advanced gastric cancer: current knowledge and future perspectives. Drugs. 2020;80(4):401–15.
Park SR, et al. Extra-gain of HER2-positive cases through HER2 reassessment in primary and metastatic sites in advanced gastric cancer with initially HER2-negative primary tumours: results of GASTric cancer HER2 reassessment study 1 (GASTHER1). Eur J Cancer. 2016;53:42–50.
Seo S, et al. Loss of HER2 positivity after anti-HER2 chemotherapy in HER2-positive gastric cancer patients: results of the GASTric cancer HER2 reassessment study 3 (GASTHER3). Gastric Cancer. 2019;22(3):527–35.
Janjigian YY, et al. Genetic predictors of response to systemic therapy in esophagogastric cancer. Cancer Discov. 2018;8(1):49–58.
Shen L. Liquid biopsy: a powerful tool to monitor trastuzumab resistance in HER2-positive metastatic gastric cancer. Cancer Commun (Lond). 2018;38(1):72.
Kwak EL, et al. Molecular heterogeneity and receptor coamplification drive resistance to targeted therapy in MET-amplified esophagogastric cancer. Cancer Discov. 2015;5(12):1271–81.
Kim J, et al. Preexisting oncogenic events impact trastuzumab sensitivity in ERBB2-amplified gastroesophageal adenocarcinoma. J Clin Invest. 2014;124(12):5145–58.
Kim ST, et al. Impact of genomic alterations on lapatinib treatment outcome and cell-free genomic landscape during HER2 therapy in HER2+ gastric cancer patients. Ann Oncol. 2018;29(4):1037–48.
Lee JY, et al. The impact of concomitant genomic alterations on treatment outcome for trastuzumab therapy in HER2-positive gastric cancer. Sci Rep. 2015;5:9289.
Sanchez-Vega F, et al. EGFR and MET amplifications determine response to HER2 Inhibition in ERBB2-amplified esophagogastric cancer. Cancer Discov. 2019;9(2):199–209.
Wang DS, et al. Liquid biopsies to track trastuzumab resistance in metastatic HER2-positive gastric cancer. Gut. 2019;68(7):1152–61.
Sampera A, et al. HER-family ligands promote acquired resistance to trastuzumab in gastric cancer. Mol Cancer Ther. 2019;18(11):2135–45.
Gambardella V, et al. NRF2 through RPS6 activation is related to anti-HER2 drug resistance in HER2-amplified gastric cancer. Clin Cancer Res. 2019;25(5):1639–49.
Nordstrom JL, et al. Anti-tumor activity and toxicokinetics analysis of MGAH22, an anti-HER2 monoclonal antibody with enhanced Fcgamma receptor binding properties. Breast Cancer Res. 2011;13(6):R123.
Shinde A, et al. Can immunotherapy replace radiotherapy in melanoma brain metastases? J Clin Oncol. 2019;37(12):1030–1.
Bang YJ, et al. First-in-human phase 1 study of margetuximab (MGAH22), an Fc-modified chimeric monoclonal antibody, in patients with HER2-positive advanced solid tumors. Ann Oncol. 2017;28(4):855–61.
Catenacci DVT, et al. Margetuximab plus pembrolizumab in patients with previously treated, HER2-positive gastro-oesophageal adenocarcinoma (CP-MGAH22-05): a single-arm, phase 1b–2 trial. Lancet Oncol. 2020;21(8):1066–76.
Catenacci DVT, et al. Margetuximab with retifanlimab as first-line therapy in HER2+/PD-L1+ unresectable or metastatic gastroesophageal adenocarcinoma: MAHOGANY cohort A. ESMO Open. 2022;7(5)
Ku G, et al. 1380P Phase (Ph) II study of zanidatamab + chemotherapy (chemo) in first-line (1L) HER2 expressing gastroesophageal adenocarcinoma (GEA). Ann Oncol. 2021;32:S1044–5.
Tabernero J, et al. HERIZON-GEA-01: Zanidatamab + chemo +/- tislelizumab for 1L treatment of HER2-positive gastroesophageal adenocarcinoma. Future Oncol. 2022;18(29):3255–66.
Zhang J, et al. First-in-human HER2-targeted bispecific antibody KN026 for the treatment of patients with HER2-positive metastatic breast cancer: results from a phase I Study. Clin Cancer Res. 2022;28(4):618–28.
Xu J, et al. A phase II study evaluating KN026 monotherapy in patients (pts) with previously treated, advanced HER2-expressing gastric or gastroesophageal junction cancers (GC/GEJC). J Clin Oncol. 2022;40(16_suppl):4040–4040.
Shen L, et al. 1210P The preliminary efficacy and safety of KN026 combined with KN046 treatment in HER2-positive locally advanced unresectable or metastatic gastric/gastroesophageal junction cancer without prior systemic treatment in a phase II study. Ann Oncol. 2022;33:S1102.
Piha-Paul S, et al. O82 A phase 1 dose escalation study of PRS-343, a HER2/4–1BB bispecific molecule, in patients with HER2-positive malignancies. J Immunother Cancer. 2020;8(Suppl 1):A1.
Google Scholar
Criscitiello C, Morganti S, Curigliano G. Antibody-drug conjugates in solid tumors: a look into novel targets. J Hematol Oncol. 2021;14(1):20.
Ogitani Y, et al. Bystander killing effect of DS-8201a, a novel anti-human epidermal growth factor receptor 2 antibody-drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci. 2016;107(7):1039–46.
Shitara K, et al. Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer. N Engl J Med. 2020;382(25):2419–30.
Yamaguchi K, et al. Trastuzumab deruxtecan in anti-human epidermal growth factor receptor 2 treatment-naive patients with human epidermal growth factor receptor 2–low gastric or gastroesophageal junction adenocarcinoma: exploratory cohort results in a phase II Trial. J Clin Oncol. 2023;41(4):816–25.
Ku GY, et al. 1205MO Updated analysis of DESTINY-Gastric02: A phase II single-arm trial of trastuzumab deruxtecan (T-DXd) in western patients (Pts) with HER2-positive (HER2+) unresectable/metastatic gastric/gastroesophageal junction (GEJ) cancer who progressed on or after trastuzumab-containing regimen. Ann Oncol. 2022;33:S1100.
Dai L, et al. Efficacy of disitamab vedotin in treating HER2 2+/FISH- gastric cancer. Onco Targets Ther. 2022;15:267–75.
Xu Y, et al. Phase I study of the recombinant humanized anti-HER2 monoclonal antibody-MMAE conjugate RC48-ADC in patients with HER2-positive advanced solid tumors. Gastric Cancer. 2021;24(4):913–25.
Peng Z, et al. Efficacy and safety of a novel anti-HER2 therapeutic antibody RC48 in patients with HER2-overexpressing, locally advanced or metastatic gastric or gastroesophageal junction cancer: a single-arm phase II study. Cancer Commun (Lond). 2021;41(11):1173–82.
Zhang Y, et al. Phase 1 multicenter, dose-expansion study of ARX788 as monotherapy in HER2-positive advanced gastric and gastroesophageal junction adenocarcinoma. Cell Rep Med. 2022;3(11): 100814.
Kulukian A, et al. Preclinical activity of HER2-selective tyrosine kinase inhibitor tucatinib as a single agent or in combination with trastuzumab or docetaxel in solid tumor models. Mol Cancer Ther. 2020;19(4):976–87.
Catenacci DVT, et al. MOUNTAINEER-02: Phase 2/3 study of tucatinib, trastuzumab, ramucirumab, and paclitaxel in previously treated HER2+ gastric or gastroesophageal junction adenocarcinoma—Trial in progress. J Clin Oncol. 2022;40(4_suppl):371.
Wu X, Huang S. HER2-specific chimeric antigen receptor-engineered natural killer cells combined with apatinib for the treatment of gastric cancer. Bull Cancer. 2019;106(11):946–58.
Smyth EC, et al. Gastric cancer. Lancet. 2020;396(10251):635–48.
Casak SJ, et al. FDA approval summary: ramucirumab for gastric cancer. Clin Cancer Res. 2015;21(15):3372–6.
Fuchs CS, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014;383(9911):31–9.
Wilke H, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15(11):1224–35.
Xu RH, et al. Efficacy and safety of weekly paclitaxel with or without ramucirumab as second-line therapy for the treatment of advanced gastric or gastroesophageal junction adenocarcinoma (RAINBOW-Asia): a randomised, multicentre, double-blind, phase 3 trial. Lancet Gastroenterol Hepatol. 2021;6(12):1015–24.
Fuchs CS, et al. Ramucirumab with cisplatin and fluoropyrimidine as first-line therapy in patients with metastatic gastric or junctional adenocarcinoma (RAINFALL): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(3):420–35.
Ohtsu A, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol. 2011;29(30):3968–76.
Pavlakis N, et al. INTEGRATE IIa: a randomised, double-blind, phase III study of regorafenib versus placebo in refractory advanced gastro-oesophageal cancer (AGOC)—a study led by the Australasian Gastro-intestinal Trials Group (AGITG). J Clin. Oncol. 2023;41(4):LBA294.
Fukuoka S, et al. Regorafenib plus nivolumab in patients with advanced gastric or colorectal cancer: an open-label, dose-escalation, and dose-expansion phase ib trial (REGONIVO, EPOC1603). J Clin Oncol. 2020;38(18):2053–61.
Kawazoe A, et al. Lenvatinib plus pembrolizumab in patients with advanced gastric cancer in the first-line or second-line setting (EPOC1706): an open-label, single-arm, phase 2 trial. Lancet Oncol. 2020;21(8):1057–65.
Li J, et al. Randomized, double-blind, placebo-controlled phase III trial of apatinib in patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J Clin Oncol. 2016;34(13):1448–54.
Kang YK, et al. Randomized phase III ANGEL study of rivoceranib (apatinib) + best supportive care (BSC) vs placebo + BSC in patients with advanced/metastatic gastric cancer who failed & 2 prior chemotherapy regimens. Ann Oncol. 2019;30:v877–8.
Zhang Y, et al. A phase Ib/II study of fruquintinib in combination with paclitaxel as the second-line therapy for advanced gastric cancer. Cancer Commun (Lond), 2022.
Sung H, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021
Sahin U, et al. Claudin-18 splice variant 2 is a pan-cancer target suitable for therapeutic antibody development. Clin Cancer Res. 2008;14(23):7624–34.
Hong JY, et al. Claudin 18.2 expression in various tumor types and its role as a potential target in advanced gastric cancer. Transl Cancer Res. 2020;9(5):3367–74.
Rohde C, et al. Comparison of Claudin 18.2 expression in primary tumors and lymph node metastases in Japanese patients with gastric adenocarcinoma. Jpn J Clin Oncol. 2019;49(9):870–6.
Sahin U, et al. FAST: a randomised phase II study of zolbetuximab (IMAB362) plus EOX versus EOX alone for first-line treatment of advanced CLDN18.2-positive gastric and gastro-oesophageal adenocarcinoma. Ann Oncol. 2021;32(5):609–19.
Singh P, Toom S, Huang Y. Anti-claudin 18.2 antibody as new targeted therapy for advanced gastric cancer. J Hematol Oncol. 2017;10(1):105.
Tureci O, et al. A multicentre, phase IIa study of zolbetuximab as a single agent in patients with recurrent or refractory advanced adenocarcinoma of the stomach or lower oesophagus: the MONO study. Ann Oncol. 2019;30(9):1487–95.
Shitara K, et al. Zolbetuximab + mFOLFOX6 as first-line (1L) treatment for patients (pts) withclaudin-18.2+ (CLDN18.2+) / HER2− locally advanced (LA) unresectable or metastatic gastric or gastroesophageal junction (mG/GEJ) adenocarcinoma: primary results from phase 3 SPOTLIGHT study. J Clin Oncol 2023;41(4_suppl): LBA292
Shitara K, et al. Zolbetuximab + CAPOX in 1L claudin-18.2+ (CLDN18.2+)/HER2− locally advanced (LA) or metastatic gastric or gastroesophageal junction (mG/GEJ) adenocarcinoma: Primary phase 3 results from GLOW. J Clin Oncol. 2023;41(36_suppl):405736–405736.
Jiang H, et al. Claudin182-specific chimeric antigen receptor engineered T cells for the treatment of gastric cancer. J Natl Cancer Inst. 2019;111(4):409–18.
Qi C, et al. Claudin18.2-specific CAR T cells in gastrointestinal cancers: phase 1 trial interim results. Nat Med. 2022;28(6):1189–98.
Helsten T, Schwaederle M, Kurzrock R. Fibroblast growth factor receptor signaling in hereditary and neoplastic disease: biologic and clinical implications. Cancer Metastasis Rev. 2015;34(3):479–96.
Yue S, et al. FGFR-TKI resistance in cancer: current status and perspectives. J Hematol Oncol. 2021;14(1):23.
Bahleda R, et al. Phase I, first-in-human study of futibatinib, a highly selective, irreversible FGFR1-4 inhibitor in patients with advanced solid tumors. Ann Oncol. 2020;31(10):1405–12.
Meric-Bernstam F, et al. Futibatinib, an irreversible FGFR1-4 inhibitor, in patients with advanced solid tumors harboring FGF/FGFR aberrations: a phase I dose-expansion study. Cancer Discov. 2022;12(2):402–15.
Lengyel CG, et al. FGFR Pathway Inhibition in Gastric Cancer: The Golden Era of an Old Target? Life (Basel), 2022;12(1)
Catenacci DVT, et al. Phase I escalation and expansion study of bemarituzumab (FPA144) in patients with advanced solid tumors and FGFR2b-selected gastroesophageal adenocarcinoma. J Clin Oncol. 2020;38(21):2418–26.
Catenacci DVT, et al. FIGHT: a randomized, double-blind, placebo-controlled, phase II study of bemarituzumab (bema) combined with modified FOLFOX6 in 1L FGFR2b+ advanced gastric/gastroesophageal junction adenocarcinoma (GC). J Clin Oncol. 2021;39(15_suppl):4010–4010.
Nagatsuma AK, et al. Expression profiles of HER2, EGFR, MET and FGFR2 in a large cohort of patients with gastric adenocarcinoma. Gastric Cancer. 2015;18(2):227–38.
Dutton SJ, et al. Gefitinib for oesophageal cancer progressing after chemotherapy (COG): a phase 3, multicentre, double-blind, placebo-controlled randomised trial. Lancet Oncol. 2014;15(8):894–904.
Petty RD, et al. Gefitinib and EGFR gene copy number aberrations in esophageal cancer. J Clin Oncol. 2017;35(20):2279–87.
Lordick F, et al. Clinical outcome according to tumor HER2 status and EGFR expression in advanced gastric cancer patients from the EXPAND study. J Clin Oncol. 2013;31(15_suppl):4021–4021.
Maron SB, et al. Targeted therapies for targeted populations: anti-EGFR Treatment for EGFR-amplified gastroesophageal adenocarcinoma. Cancer Discov. 2018;8(6):696–713.
Raj S, et al. Molecular mechanism(s) of regulation(s) of c-MET/HGF signaling in head and neck cancer. Mol Cancer. 2022;21(1):31.
Shah MA, et al. A randomized phase II study of FOLFOX with or without the MET inhibitor onartuzumab in advanced adenocarcinoma of the stomach and gastroesophageal junction. Oncologist. 2016;21(9):1085–90.
Catenacci DVT, et al. Rilotumumab plus epirubicin, cisplatin, and capecitabine as first-line therapy in advanced MET-positive gastric or gastro-oesophageal junction cancer (RILOMET-1): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18(11):1467–82.
Doi T, et al. A phase 3, multicenter, randomized, double-blind, placebo-controlled study of rilotumumab in combination with cisplatin and capecitabine (CX) as first-line therapy for Asian patients (pts) with advanced MET-positive gastric or gastroesophageal junction (G/GEJ) adenocarcinoma: the RILOMET-2 trial. J Clin Oncol. 2015;33(3_suppl): TPS226
Kang YK, et al. A phase II trial of a selective c-Met inhibitor tivantinib (ARQ 197) monotherapy as a second- or third-line therapy in the patients with metastatic gastric cancer. Invest New Drugs. 2014;32(2):355–61.
Hong DS, et al. Phase I Study of AMG 337, a highly selective small-molecule MET inhibitor, in patients with advanced solid tumors. Clin Cancer Res. 2019;25(8):2403–13.
Shah MA, et al. Phase II study evaluating 2 dosing schedules of oral foretinib (GSK1363089), cMET/VEGFR2 inhibitor, in patients with metastatic gastric cancer. PLoS ONE. 2013;8(3): e54014.
Luo H, et al. Real-time artificial intelligence for detection of upper gastrointestinal cancer by endoscopy: a multicentre, case-control, diagnostic study. Lancet Oncol. 2019;20(12):1645–54.
Chen ZH, et al. Artificial intelligence for assisting cancer diagnosis and treatment in the era of precision medicine. Cancer Commun (Lond). 2021;41(11):1100–15.
Leal A, et al. White blood cell and cell-free DNA analyses for detection of residual disease in gastric cancer. Nat Commun. 2020;11(1):525.
Maron SB, et al. Circulating tumor DNA sequencing analysis of gastroesophageal adenocarcinoma. Clin Cancer Res. 2019;25(23):7098–112.
Jin Y, et al. The predicting role of circulating tumor DNA landscape in gastric cancer patients treated with immune checkpoint inhibitors. Mol Cancer. 2020;19(1):154.
Wang Y, et al. Immune checkpoint modulators in cancer immunotherapy: recent advances and emerging concepts. J Hematol Oncol. 2022;15(1):111.
Upadhaya S, et al. Combinations take centre stage in PD1/PDL1 inhibitor clinical trials. Nat Rev Drug Discov. 2021;20(3):168–9.
Zhao H, et al. Emerging immunological strategies: recent advances and future directions. Front Med. 2021;15(6):805–28.
Download references
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 82203678 to Y.H.), the Science and Technology Program of Guangdong (Grant No. 2019B020227002 to R.-H.X.), the CAMS Innovation Fund for Medical Sciences (Grant No. 2019-I2M-5-036 to R.-H.X.).
Author information
Wen-Long Guan and Ye He have contributed equally.
Authors and Affiliations
Department of Medical Oncology, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Sun Yat-Sen University Cancer Center, 651 Dongfeng Road East, Guangzhou, 510060, People’s Republic of China
Wen-Long Guan, Ye He & Rui-Hua Xu
Research Unit of Precision Diagnosis and Treatment for Gastrointestinal Cancer, Chinese Academy of Medical Sciences, Guangzhou, 510060, People’s Republic of China
You can also search for this author in PubMed Google Scholar
Contributions
Rui-Hua Xu designed this review. Rui-Hua Xu, Wen-Long Guan, and Ye He drafted the manuscript and prepared the figures. Rui-Hua Xu, Wen-Long Guan, and Ye He collected the related references and participated in the discussion. All authors contributed to this manuscript and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Rui-Hua Xu .
Ethics declarations
Ethical approval and consent to participate, competing interests.
All authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Guan, WL., He, Y. & Xu, RH. Gastric cancer treatment: recent progress and future perspectives. J Hematol Oncol 16 , 57 (2023). https://doi.org/10.1186/s13045-023-01451-3
Download citation
Received : 13 March 2023
Accepted : 10 May 2023
Published : 27 May 2023
DOI : https://doi.org/10.1186/s13045-023-01451-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Immunotherapy
- Targeted therapy
Journal of Hematology & Oncology
ISSN: 1756-8722
- General enquiries: [email protected]

No matter what phase of stomach cancer, patients and caregivers can always find Hope.

From fundraisers to social media and more you have lots of options to help!

Sometimes money is the easiest way to give. But spreading awareness is a greatly needed too.

Hope bridges the gaps between the medical community and gastric cancer patients.
There's Always Hope
The help you need is here. We are warriors in the fight against stomach cancer. Patients, caregivers, loved ones, and the medical community.

Stomach Cancer Patient Empowerment Summit
Our 2nd Stomach Cancer Patient Empowerment Summit and 1st Gastric Cancer Advocacy on Capitol Hill Day was from May 5 to May 7, 2024 in DC. The theme of these events was Your Story Matters. We came together as a community of patients, caregivers, and advocates in our shared journey.

Hope In A Bag: Newly Diagnosed Patient Resources
This invaluable document bundle provides a customizable roadmap to fighting stomach cancer. Order yours now – there’s no charge.
Learn About Stomach Cancer
Stomach cancer is also known as gastric cancer. In the US it’s often misdiagnosed. Here’s more about the types, stages, and testing.
No organization supports stomach cancer patients like Hope for Stomach Cancer. We create and facilitate programs enabling those affected by stomach cancer to take actionable steps and live the best possible life through each phase of the disease.

Exercise, therapy and diet can all improve life during cancer treatment and boost survival. Here’s how
Professor of Exercise Medicine, Edith Cowan University
Disclosure statement
Rob Newton receives funding from NHMRC, Cancer Council WA, Cancer Australia, World Cancer Research Foundation.
Edith Cowan University provides funding as a member of The Conversation AU.
View all partners
With so many high-profile people diagnosed with cancer we are confronted with the stark reality the disease can strike any of us at any time. There are also reports certain cancers are increasing among younger people in their 30s and 40s.
On the positive side, medical treatments for cancer are advancing very rapidly. Survival rates are improving greatly and some cancers are now being managed more as long-term chronic diseases rather than illnesses that will rapidly claim a patient’s life.
The mainstays of cancer treatment remain surgery, chemotherapy, radiation therapy, immunotherapy, targeted therapy and hormone therapy. But there are other treatments and strategies – “adjunct” or supportive cancer care – that can have a powerful impact on a patient’s quality of life, survival and experience during cancer treatment.
Keep moving if you can
Physical exercise is now recognised as a medicine . It can be tailored to the patient and their health issues to stimulate the body and build an internal environment where cancer is less likely to flourish . It does this in a number of ways.
Exercise provides a strong stimulus to our immune system, increasing the number of cancer-fighting immune cells in our blood circulation and infusing these into the tumour tissue to identify and kill cancer cells .
Our skeletal muscles (those attached to bone for movement) release signalling molecules called myokines . The larger the muscle mass, the more myokines are released – even when a person is at rest. However, during and immediately after bouts of exercise, a further surge of myokines is secreted into the bloodstream. Myokines attach to immune cells, stimulating them to be better “hunter-killers”. Myokines also signal directly to cancer cells slowing their growth and causing cell death .
Exercise can also greatly reduce the side effects of cancer treatment such as fatigue, muscle and bone loss, and fat gain. And it reduces the risk of developing other chronic diseases such as heart disease and type 2 diabetes. Exercise can maintain or improve quality of life and mental health for patients with cancer .
Emerging research evidence indicates exercise might increase the effectiveness of mainstream treatments such as chemotherapy and radiation therapy . Exercise is certainly essential for preparing the patient for any surgery to increase cardio-respiratory fitness, reduce systemic inflammation, and increase muscle mass, strength and physical function, and then rehabilitating them after surgery .
These mechanisms explain why cancer patients who are physically active have much better survival outcomes with the relative risk of death from cancer reduced by as much as 40–50% .
Mental health helps
The second “tool” which has a major role in cancer management is psycho-oncology . It involves the psychological, social, behavioural and emotional aspects of cancer for not only the patient but also their carers and family. The aim is to maintain or improve quality of life and mental health aspects such as emotional distress, anxiety, depression, sexual health, coping strategies, personal identity and relationships.
Supporting quality of life and happiness is important on their own, but these barometers can also impact a patient’s physical health, response to exercise medicine, resilience to disease and to treatments.
If a patient is highly distressed or anxious, their body can enter a flight or fight response. This creates an internal environment that is actually supportive of cancer progression through hormonal and inflammatory mechanisms . So it’s essential their mental health is supported.
Putting the good things in: diet
A third therapy in the supportive cancer care toolbox is diet. A healthy diet can support the body to fight cancer and help it tolerate and recover from medical or surgical treatments.
Inflammation provides a more fertile environment for cancer cells . If a patient is overweight with excessive fat tissue then a diet to reduce fat which is also anti-inflammatory can be very helpful. This generally means avoiding processed foods and eating predominantly fresh food, locally sourced and mostly plant based.

Muscle loss is a side effect of all cancer treatments . Resistance training exercise can help but people may need protein supplements or diet changes to make sure they get enough protein to build muscle. Older age and cancer treatments may reduce both the intake of protein and compromise absorption so supplementation may be indicated .
Depending on the cancer and treatment, some patients may require highly specialised diet therapy. Some cancers such as pancreatic, stomach, esophageal, and lung cancer can cause rapid and uncontrolled drops in body weight. This is called cachexia and needs careful management .
Other cancers and treatments such as hormone therapy can cause rapid weight gain. This also needs careful monitoring and guidance so that, when a patient is clear of cancer, they are not left with higher risks of other health problems such as cardiovascular disease and metabolic syndrome (a cluster of conditions that boost your risk of heart disease, stroke and type 2 diabetes).
Working as a team
These are three of the most powerful tools in the supportive care toolbox for people with cancer. None of them are “cures” for cancer, alone or together. But they can work in tandem with medical treatments to greatly improve outcomes for patients.
If you or someone you care about has cancer, national and state cancer councils and cancer-specific organisations can provide support.
For exercise medicine support it is best to consult with an accredited exercise physiologist , for diet therapy an accredited practising dietitian and mental health support with a registered psychologist . Some of these services are supported through Medicare on referral from a general practitioner.
For free and confidential cancer support call the Cancer Council on 13 11 20.
- Mental health
- Cancer treatment
- Consumer health

Research Fellow

Senior Research Fellow - Women's Health Services

Lecturer / Senior Lecturer - Marketing

Assistant Editor - 1 year cadetship

Executive Dean, Faculty of Health

IMAGES
VIDEO
COMMENTS
What are the symptoms of stomach cancer, and when should you see a doctor about them? Here, surgical oncologist and gastrointestinal cancer specialist Paul Mansfield, M.D., weighs in, and six survivors share how they knew they had stomach cancer.
Recognizing symptoms and risk factors is essential, yet it's the personal narratives of individuals who have directly confronted stomach cancer that genuinely highlight the importance of early detection and proactive health management. Robert, a 60-year-old stomach cancer survivor in Land O' Lakes, FL, was a surgery patient of Dr. Ross and her team who shares his story here.
Treatment given after surgery, to lower the risk that the cancer will come back, is called adjuvant therapy. After the doctor removes all the cancer that can be seen, some patients may be given chemotherapy, radiation therapy, or both to kill any cancer cells that are left.
Are you newly diagnosed with gastric (stomach) cancer or a caregiver? You are not alone. Resources and support are available. Gastric Cancer Foundation was established to provide information and an understanding community for people with gastric cancer and their loved ones.
If you or someone you love has stomach cancer, knowing what to expect can help. Find out more information on stomach cancer, including symptoms and treatment.
In honor of Stomach Cancer Awareness Month Martha Raymond, Executive Director of the GI Cancers Alliance, interviews Steve Melen, a Stomach Cancer survivor and patient advocate, about his story with Stomach Cancer when he was diagnosed at 37 years old, having a new baby, a new job and other pressures in his life. Steve even features his new book, Killer Graces: My path from pain to power and ...
Supporting patient education initiatives and innovative research studies to improve treatments and ultimately find a cure for gastric cancer.
Treatment of stomach cancer (also known as gastric cancer) depends largely on where the cancer is in the stomach and how far it has spread. But other factors, such as a person's age, overall health, and preferences, can be important as well.
Gastric cancer treatment options depend on extent of disease and may include radical surgery, chemotherapy, radiation, and immunotherapy. Get detailed information about the diagnosis, treatment, and prognosis of newly diagnosed and recurrent gastric cancer in this clinician summary.
Gastric cancer remains a major unmet clinical problem with over 1 million new cases in 2018 worldwide. It is the fourth most commonly occurring cancer in men and the seventh most commonly occurring cancer in women. A major fraction of gastric cancer has ...
Before surgery, I completed scans, and the doctors couldn't find any signs of cancer. But to be safe, I underwent a total gastrectomy, which totally removes the stomach and reattaches the small intestines to the esophagus. After surgery, my care team determined that the cancer had not spread to my lymph nodes.
Gastric cancer often begins in the inner lining of the stomach (the mucosa), grows into the stomach wall and may spread to other areas through the lymph drainage and blood vessels.
Learn about the signs, symptoms and causes of stomach cancer (gastric cancer). This condition happens when a growth of cells starts in the stomach.
We are proud to partner with Smart Patients to offer a secure online peer-to-peer support community where people affected by gastric cancer can share advice, resources, and support. Your well-being is our priority. Our forum is a confidential space, limited to those who have been specifically impacted by gastric cancer.
Stomach cancer is also called gastric cancer. There are many types that if diagnosed at an early stage, have a good chance of being cured. Written by a GP.
Gastric cancer is the fifth most frequently diagnosed cancer and the third leading cause of cancer deaths worldwide. The only potentially curative treatment approach for patients with gastric cancer is surgical resection with adequate lymphadenectomy. Regrettably, patients with an unresectable, locally advanced, or metastatic disease can only be offered life-prolonging palliative therapy ...
Patients with newly diagnosed gastric cancer often present with an upper endoscopy report performed for symptoms, including dyspepsia and reflux, but also with symptoms or signs that may indicate advanced disease, such as dysphagia, weight loss, gastrointestinal bleeding, anemia, and emesis. 13 Clear measurements of the extent of the primary tumor are often lacking, and repeat endoscopy with ...
A Patient's Journey: Gastric Stomach Cancer PatientPoint Studios
Gastric and gastro-oesophageal cancer is a leading cause of cancer-related death worldwide with a poor prognosis. This Review provides a comprehensive overview of current treatment strategies set ...
The standardization of surgical treatment of gastric cancer in accordance with the patient's profile is of decisive importance for a better outcome. This review aims to summarize the current standards in the surgical treatment of gastric cancer. Keywords: gastric cancer, surgery, lymphadenectomy, survival.
Gastric cancer (GC) is one of the most common malignancies worldwide. Most patients are diagnosed at advanced stages due to the subtle symptoms of earlier disease and the low rate of regular screening. Systemic therapies for GC, including chemotherapy, targeted therapy and immunotherapy, have evolved significantly in the past few years. For resectable GC, perioperative chemotherapy has become ...
Our 2nd Stomach Cancer Patient Empowerment Summit and 1st Gastric Cancer Advocacy on Capitol Hill Day was from May 5 to May 7, 2024 in DC. The theme of these events was Your Story Matters. We came together as a community of patients, caregivers, and advocates in our shared journey.
Patients with advanced or metastatic gastric cancer often suffer from malnutrition, which can have an impact on quality of life, increase the toxicity of chemotherapy and reduce overall survival. Options available to the clinician to manage a patient's nutritional status include screening and assessment of malnutrition at diagnosis, monitoring during the 'cancer journey', early detection ...
When you or someone you love has GI cancer, you may have many questions. You may wonder: Are gastrointestinal cancers curable? Fortunately, new advances in gastrointestinal cancer treatment, including stomach cancer treatment, offer options and hope.
The mainstays of cancer treatment remain surgery, chemotherapy, radiation, immunotherapy, targeted therapy, and hormone therapy. But exercise, psychological support and diet can be powerful adjuncts.