Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 15 January 2024
Parkinson disease psychosis: from phenomenology to neurobiological mechanisms
- Javier Pagonabarraga ORCID: orcid.org/0000-0002-3248-704X 1 , 2 , 3 , 4 ,
- Helena Bejr-Kasem 1 , 2 , 3 , 4 ,
- Saul Martinez-Horta 1 , 2 , 3 , 4 &
- Jaime Kulisevsky ORCID: orcid.org/0000-0003-4870-1431 1 , 2 , 3 , 4
Nature Reviews Neurology volume 20 , pages 135–150 ( 2024 ) Cite this article
1722 Accesses
29 Altmetric
Metrics details
- Parkinson's disease
- Psychiatric disorders
Parkinson disease (PD) psychosis (PDP) is a spectrum of illusions, hallucinations and delusions that are associated with PD throughout its disease course. Psychotic phenomena can manifest from the earliest stages of PD and might follow a continuum from minor hallucinations to structured hallucinations and delusions. Initially, PDP was considered to be a complication associated with dopaminergic drug use. However, subsequent research has provided evidence that PDP arises from the progression of brain alterations caused by PD itself, coupled with the use of dopaminergic drugs. The combined dysfunction of attentional control systems, sensory processing, limbic structures, the default mode network and thalamocortical connections provides a conceptual framework to explain how new incoming stimuli are incorrectly categorized, and how aberrant hierarchical predictive processing can produce false percepts that intrude into the stream of consciousness. The past decade has seen the publication of new data on the phenomenology and neurobiological basis of PDP from the initial stages of the disease, as well as the neurotransmitter systems involved in PDP initiation and progression. In this Review, we discuss the latest clinical, neuroimaging and neurochemical evidence that could aid early identification of psychotic phenomena in PD and inform the discovery of new therapeutic targets and strategies.
Parkinson disease (PD) psychosis (PDP) comprises a spectrum of illusions, hallucinations and delusions that are associated with PD throughout its course.
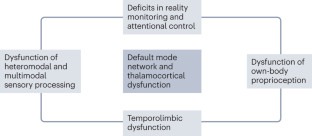
PDP is attributable not only to the use of dopaminergic drugs but also to inherent disruptions linked to the disease, which lead to dysfunction of neural systems governing visual perception, multimodal sensory integration, reality monitoring and attention.
Both minor and structured hallucinations in PD are associated with a pattern of cortical atrophy that includes the cuneus, precuneus, middle occipital gyrus, lingual and fusiform gyri, supramarginal gyrus, angular gyrus, anterior cingulate cortex, hippocampal regions and thalamus.
Functional neuroimaging studies indicate that PDP is associated with failure of top-down processing of attentional networks, aberrant coupling of the default mode network with visual networks and disconnection between the thalamus and posterior brain areas, leading to aberrant disinhibition of the default mode network.
Cortical cholinergic denervation and elevated levels of 5-HT 2A serotonergic receptor binding in the ventral visual pathway, medial orbitofrontal cortex and insula have prominent roles in the development of visual hallucinations.
An important advance in the treatment of PDP has been the development of drugs that reduce the activity of cortical postsynaptic 5-HT 2A receptors, of which pimavanserin is the most notable.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
195,33 € per year
only 16,28 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others

Structural pharmacology and therapeutic potential of 5-methoxytryptamines

The Amyloid-β Pathway in Alzheimer’s Disease

ffytche, D. H. et al. The psychosis spectrum in Parkinson disease. Nat. Rev. Neurol. 13 , 81–95 (2017).
Article PubMed PubMed Central Google Scholar
Diederich, N. J., Fénelon, G., Stebbins, G. & Goetz, C. G. Hallucinations in Parkinson disease. Nat. Rev. Neurol. 5 , 331–342 (2009).
Article CAS PubMed Google Scholar
Damasio, A. R., Lobo-Antunes, J. & Macedo, C. Psychiatric aspects in Parkinsonism treated with L-dopa. J. Neurol. Neurosurg. Psychiatry 34 , 502–507 (1971).
Article CAS PubMed PubMed Central Google Scholar
Moskovitz, C., Moses, H. 3rd & Klawans, H. L. Levodopa-induced psychosis: a kindling phenomenon. Am. J. Psychiatry 135 , 669–675 (1978).
Rinne, U. K., Sonninen, V. & Marttila, R. Dopaminergic agonist effects on Parkinsonian clinical features and brain monamine metabolism. Adv. Neurol. 9 , 383–392 (1975).
CAS PubMed Google Scholar
Parkes, J. D. et al. Bromocriptine treatment in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 39 , 184–193 (1976).
Holroyd, S. Prospective study of hallucinations and delusions in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 70 , 734–738 (2001).
Goetz, C. G., Leurgans, S., Pappert, E. J., Raman, R. & Stemer, A. B. Prospective longitudinal assessment of hallucinations in Parkinson’s disease. Neurology 57 , 2078–2082 (2001).
Williams, D. R. & Lees, A. J. Visual hallucinations in the diagnosis of idiopathic Parkinson’s disease: a retrospective autopsy study. Lancet Neurol. 4 , 605–610 (2005).
Article PubMed Google Scholar
Fénelon, G. Hallucinations in Parkinson’s disease: prevalence, phenomenology and risk factors. Brain 123 , 733–745 (2000).
Fénelon, G., Goetz, C. G. & Karenberg, A. Hallucinations in Parkinson disease in the prelevodopa era. Neurology 66 , 93–98 (2006).
Biousse, V. et al. Ophthalmologic features of Parkinson’s disease. Neurology 62 , 177–180 (2004).
Ibarretxe-Bilbao, N. et al. Differential progression of brain atrophy in Parkinson’s disease with and without visual hallucinations. J. Neurol. Neurosurg. Psychiatry 81 , 650–657 (2010).
Goldman, J. G. et al. Visuoperceptive region atrophy independent of cognitive status in patients with Parkinson’s disease with hallucinations. Brain 137 , 849–859 (2014).
Meppelink, A. M. et al. Impaired visual processing preceding image recognition in Parkinson’s disease patients with visual hallucinations. Brain 132 , 2980–2993 (2009).
Pagonabarraga, J. et al. Neural correlates of minor hallucinations in non-demented patients with Parkinson’s disease. Parkinsonism Relat. Disord. 20 , 290–296 (2014).
Lenka, A., Pagonabarraga, J., Pal, P. K., Bejr-Kasem, H. & Kulisvesky, J. Minor hallucinations in Parkinson disease. Neurology 93 , 259–266 (2019).
Fénelon, G., Soulas, T., de Langavant, L. C., Trinkler, I. & Bachoud-Levi, A.-C. Feeling of presence in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 82 , 1219–1224 (2011).
Pagonabarraga, J. et al. Minor hallucinations occur in drug-naive Parkinson’s disease patients, even from the premotor phase. Mov. Disord. 31 , 45–52 (2016).
Ravina, B. et al. Diagnostic criteria for psychosis in Parkinson’s disease: report of an NINDS, NIMH work group. Mov. Disord. 22 , 1061–1068 (2007).
Fénelon, G., Soulas, T., Zenasni, F. & de Langavant, L. C. The changing face of Parkinson’s disease-associated psychosis: a cross-sectional study based on the new NINDS–NIMH criteria. Mov. Disord. 25 , 763–766 (2010).
Zhong, M. et al. Prevalence and risk factors for minor hallucinations in patients with Parkinson’s disease. Behav. Neurol. 2021 , 3469706 (2021).
Wood, R. A., Hopkins, S. A., Moodley, K. K. & Chan, D. Fifty percent prevalence of extracampine hallucinations in Parkinson’s disease patients. Front. Neurol. 6 , 263 (2015).
Bejr‐Kasem, H. et al. Minor hallucinations reflect early gray matter loss and predict subjective cognitive decline in Parkinson’s disease. Eur. J. Neurol. 28 , 438–447 (2021).
Doé de Maindreville, A., Fénelon, G. & Mahieux, F. Hallucinations in Parkinson’s disease: a follow‐up study. Mov. Disord. 20 , 212–217 (2005).
Article Google Scholar
Sasaki, C. et al. Visual illusions in Parkinson’s disease: an interview survey of symptomatology. Psychogeriatrics 22 , 38–48 (2022).
Nishio, Y. et al. Defining visual illusions in Parkinson’s disease: kinetopsia and object misidentification illusions. Parkinsonism Relat. Disord. 55 , 111–116 (2018).
Kulisevsky, J., Pagonabarraga, J., Pascual-Sedano, B., García-Sánchez, C. & Gironell, A. Prevalence and correlates of neuropsychiatric symptoms in Parkinson’s disease without dementia. Mov. Disord. 23 , 1889–1896 (2008).
Gibson, G. et al. Frequency, prevalence, incidence and risk factors associated with visual hallucinations in a sample of patients with Parkinson’s disease: a longitudinal 4-year study. Int. J. Geriatr. Psychiatry 28 , 626–631 (2013).
Stang, C. D. et al. Incidence, prevalence, and mortality of psychosis associated with Parkinson’s disease (1991–2010). J. Parkinsons Dis. 12 , 1319–1327 (2022).
Aarsland, D., Ballard, C., Larsen, J. P. & McKeith, I. A comparative study of psychiatric symptoms in dementia with Lewy bodies and Parkinson’s disease with and without dementia. Int. J. Geriatr. Psychiatry 16 , 528–536 (2001).
Chendo, I. et al. High frequency of psychosis in late-stage Parkinson’s disease. Clin. Park. Relat. Disord. 5 , 100119 (2021).
PubMed PubMed Central Google Scholar
Forsaa, E. B. et al. A 12-year population-based study of psychosis in Parkinson disease. Arch. Neurol. 67 , 996–1001 (2010).
O’Brien, J. et al. Visual hallucinations in neurological and ophthalmological disease: pathophysiology and management. J. Neurol. Neurosurg. Psychiatry 91 , 512–519 (2020).
Goetz, C. G. & Stebbins, G. T. Mortality and hallucinations in nursing home patients with advanced Parkinson’s disease. Neurology 45 , 669–671 (1995).
Montagnese, M. et al. Cognition, hallucination severity and hallucination-specific insight in neurodegenerative disorders and eye disease. Cogn. Neuropsychiatry 27 , 105–121 (2022).
Mosimann, U. P. et al. Characteristics of visual hallucinations in Parkinson disease dementia and dementia with Lewy bodies. Am. J. Geriatr. Psychiatry 14 , 153–160 (2006).
Onofrj, M., Thomas, A. & Bonanni, L. New approaches to understanding hallucinations in Parkinson’s disease: phenomenology and possible origins. Expert Rev. Neurother. 7 , 1731–1750 (2007).
Eversfield, C. L. & Orton, L. D. Auditory and visual hallucination prevalence in Parkinson’s disease and dementia with Lewy bodies: a systematic review and meta-analysis. Psychol. Med. 49 , 2342–2353 (2019).
Fénelon, G., Thobois, S., Bonnet, A.-M., Broussolle, E. & Tison, F. Tactile hallucinations in Parkinson’s disease. J. Neurol. 249 , 1699–1703 (2002).
Goetz, C. G., Vogel, C., Tanner, C. M. & Stebbins, G. T. Early dopaminergic drug-induced hallucinations in parkinsonian patients. Neurology 51 , 811–814 (1998).
Goetz, C. G., Stebbins, G. T. & Ouyang, B. Visual plus nonvisual hallucinations in Parkinson’s disease: development and evolution over 10 years. Mov. Disord. 26 , 2196–2200 (2011).
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (American Psychiatric Association Publishing, 2022).
Factor, S. A. et al. Cognitive correlates of hallucinations and delusions in Parkinson’s disease. J. Neurol. Sci. 347 , 316–321 (2014).
Warren, N., O’Gorman, C., Hume, Z., Kisely, S. & Siskind, D. Delusions in Parkinson’s disease: a systematic review of published cases. Neuropsychol. Rev. 28 , 310–316 (2018).
Stefanis, N. et al. Isolated delusional syndrome in Parkinson’s disease. Parkinsonism Relat. Disord. 16 , 550–552 (2010).
Kiziltan, G., Özekmekçi, S., Ertan, S., Ertan, T. & Erginöz, E. Relationship between age and subtypes of psychotic symptoms in Parkinson’s disease. J. Neurol. 254 , 448–452 (2007).
Poletti, M. et al. Dopamine agonists and delusional jealousy in Parkinson’s disease: a cross-sectional prevalence study. Mov. Disord. 27 , 1679–1682 (2012).
De Michele, G. et al. Othello syndrome in Parkinson’s disease: a systematic review and report of a case series. Neurol. Sci. 42 , 2721–2729 (2021).
Article PubMed PubMed Central ADS Google Scholar
Hashimoto, M., Sakamoto, S. & Ikeda, M. Clinical features of delusional jealousy in elderly patients with dementia. J. Clin. Psychiatry 76 , 691–695 (2015).
Ballard, C. et al. Psychiatric morbidity in dementia with Lewy bodies: a prospective clinical and neuropathological comparative study with Alzheimer’s disease. Am. J. Psychiatry 156 , 1039–1045 (1999).
Perini, G. et al. Misidentification delusions. Alzheimer Dis. Assoc. Disord. 30 , 331–337 (2016).
Christodoulou, G. N., Margariti, M., Kontaxakis, V. P. & Christodoulou, N. G. The delusional misidentification syndromes: strange, fascinating, and instructive. Curr. Psychiatry Rep. 11 , 185–189 (2009).
Pagonabarraga, J. et al. A prospective study of delusional misidentification syndromes in Parkinson’s disease with dementia. Mov. Disord. 23 , 443–448 (2008).
Roane, D. M., Rogers, J. D., Robinson, J. H. & Feinberg, T. E. Delusional misidentification in association with parkinsonism. J. Neuropsychiatry Clin. Neurosci. 10 , 194–198 (1998).
Hermanowicz, N. Delusional misidentification in Parkinson’s disease: report of two cases and a review. Postgrad. Med. 130 , 280–283 (2018).
Moro, A., Munhoz, R. P., Moscovich, M., Arruda, W. O. & Teive, H. A. G. Delusional misidentification syndrome and other unusual delusions in advanced Parkinson’s disease. Parkinsonism Relat. Disord. 19 , 751–754 (2013).
Nagahama, Y., Okina, T., Suzuki, N. & Matsuda, M. Neural correlates of psychotic symptoms in dementia with Lewy bodies. Brain 133 , 557–567 (2010).
Mentis, M. J. et al. Abnormal brain glucose metabolism in the delusional misidentification syndromes: a positron emission tomography study in Alzheimer disease. Biol. Psychiatry 38 , 438–449 (1995).
Weilnhammer, V. A., Stuke, H., Sterzer, P. & Schmack, K. The neural correlates of hierarchical predictions for perceptual decisions. J. Neurosci. 38 , 5008–5021 (2018).
Pezzoli, S. et al. Neuroanatomical and cognitive correlates of visual hallucinations in Parkinson’s disease and dementia with Lewy bodies: voxel-based morphometry and neuropsychological meta-analysis. Neurosci. Biobehav. Rev. 128 , 367–382 (2021).
Aarsland, D. et al. Parkinson disease-associated cognitive impairment. Nat. Rev. Dis. Primers 7 , 47 (2021).
Martinez-Horta, S. & Kulisevsky, J. Mild cognitive impairment in Parkinson’s disease. J. Neural Transm. 126 , 897–904 (2019).
Anang, J. B. et al. Predictors of dementia in Parkinson disease: a prospective cohort study. Neurology 83 , 1253–1260 (2014).
Goetz, C. G., Fan, W., Leurgans, S., Bernard, B. & Stebbins, G. T. The malignant course of “benign hallucinations” in Parkinson disease. Arch. Neurol. 63 , 713–716 (2006).
Llebaria, G. et al. Neuropsychological correlates of mild to severe hallucinations in Parkinson’s disease. Mov. Disord. 25 , 2785–2791 (2010).
Bronnick, K., Emre, M., Tekin, S., Haugen, S. B. & Aarsland, D. Cognitive correlates of visual hallucinations in dementia associated with Parkinson’s disease. Mov. Disord. 26 , 824–829 (2011).
Thomas, G. E. C. et al. Changes in both top-down and bottom-up effective connectivity drive visual hallucinations in Parkinson’s disease. Brain Commun. 5 , fcac329 (2023).
Serre, T., Oliva, A. & Poggio, T. A feedforward architecture accounts for rapid categorization. Proc. Natl Acad. Sci. USA 104 , 6424–6429 (2007).
Article CAS PubMed PubMed Central ADS Google Scholar
Blanke, O. Multisensory brain mechanisms of bodily self-consciousness. Nat. Rev. Neurosci. 13 , 556–571 (2012).
Bernasconi, F. et al. Robot-induced hallucinations in Parkinson’s disease depend on altered sensorimotor processing in fronto-temporal network. Sci. Transl. Med. 13 , eabc8362 (2021).
Bar, M. A cortical mechanism for triggering top-down facilitation in visual object recognition. J. Cogn. Neurosci. 15 , 600–609 (2003).
Collerton, D., Perry, E. & McKeith, I. Why people see things that are not there: a novel perception and attention deficit model for recurrent complex visual hallucinations. Behav. Brain Sci. 28 , 737–757 (2005).
Shine, J. M. et al. The role of dysfunctional attentional control networks in visual misperceptions in Parkinson’s disease. Hum. Brain Mapp. 35 , 2206–2219 (2014).
Schendan, H. E. & Ganis, G. Top-down modulation of visual processing and knowledge after 250 ms supports object constancy of category decisions. Front. Psychol. 6 , 1289 (2015).
Schendan, H. E. & Maher, S. M. Object knowledge during entry-level categorization is activated and modified by implicit memory after 200 ms. Neuroimage 44 , 1423–1438 (2009).
Bejr-Kasem, H. et al. The role of attentional control over interference in minor hallucinations in Parkinson’s disease. Parkinsonism Relat. Disord. 102 , 101–107 (2022).
Barnes, J., Boubert, L., Harris, J., Lee, A. & David, A. S. Reality monitoring and visual hallucinations in Parkinson’s disease. Neuropsychologia 41 , 565–574 (2003).
Johnson, M. K., Hashtroudi, S. & Lindsay, D. S. Source monitoring. Psychol. Bull. 114 , 3–28 (1993).
Muller, A. J., Shine, J. M., Halliday, G. M. & Lewis, S. J. G. Visual hallucinations in Parkinson’s disease: theoretical models. Mov. Disord. 29 , 1591–1598 (2014).
Collerton, D. et al. Understanding visual hallucinations: a new synthesis. Neurosci. Biobehav. Rev. 150 , 105208 (2023).
Geddes, M. R. et al. Altered functional connectivity in lesional peduncular hallucinosis with REM sleep behavior disorder. Cortex 74 , 96–106 (2016).
Nishio, Y. et al. Deconstructing psychosis and misperception symptoms in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 88 , 722–729 (2017).
Bejr-Kasem, H. et al. Disruption of the default mode network and its intrinsic functional connectivity underlies minor hallucinations in Parkinson’s disease. Mov. Disord. 34 , 78–86 (2019).
Zhong, M. et al. Aberrant gray matter volume and functional connectivity in Parkinson’s disease with minor hallucination. Front. Aging Neurosci. 14 , 923560 (2022).
Nagahama, Y. et al. Classification of psychotic symptoms in dementia with Lewy bodies. Am. J. Geriatr. Psychiatry 15 , 961–967 (2007).
Vignando, M. et al. Mapping brain structural differences and neuroreceptor correlates in Parkinson’s disease visual hallucinations. Nat. Commun. 13 , 519 (2022).
Barrett, M. J., Blair, J. C., Sperling, S. A., Smolkin, M. E. & Druzgal, T. J. Baseline symptoms and basal forebrain volume predict future psychosis in early Parkinson disease. Neurology 90 , e1618–e1626 (2018).
Lenka, A. et al. Hippocampal subfield atrophy in patients with Parkinson’s disease and psychosis. J. Neural Transm. 125 , 1361–1372 (2018).
Ramírez-Ruiz, B. et al. Cerebral atrophy in Parkinson’s disease patients with visual hallucinations. Eur. J. Neurol. 14 , 750–756 (2007).
Shin, S. et al. Neuroanatomical substrates of visual hallucinations in patients with non-demented Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 83 , 1155–1161 (2012).
Watanabe, H. et al. Cortical and subcortical brain atrophy in Parkinson’s disease with visual hallucination. Mov. Disord. 28 , 1732–1736 (2013).
Okada, K., Suyama, N., Oguro, H., Yamaguchi, S. & Kobayashi, S. Medication-induced hallucination and cerebral blood flow in Parkinson’s disease. J. Neurol. 246 , 365–368 (1999).
Stebbins, G. T. et al. Altered cortical visual processing in PD with hallucinations: an fMRI study. Neurology 63 , 1409–1416 (2004).
Oishi, N. et al. Regional cerebral blood flow in Parkinson disease with nonpsychotic visual hallucinations. Neurology 65 , 1708–1715 (2011).
Matsui, H. et al. Hypoperfusion of the visual pathway in parkinsonian patients with visual hallucinations. Mov. Disord. 21 , 2140–2144 (2006).
Boecker, H., Ceballos-Baumann, A. O., Volk, D. & Conrad, B. Metabolic alterations in patients with Parkinson disease and visual hallucinations. Arch. Neurol. 64 , 984–988 (2015).
Goetz, C. G., Vaughan, C. L., Goldman, J. G. & Stebbins, G. T. I finally see what you see: Parkinson’s disease visual hallucinations captured with functional neuroimaging. Mov. Disord. 29 , 115–117 (2014).
Gasca-Salas, C., Clavero, P., García-García, D., Obeso, J. A. & Rodríguez-Oroz, M. C. Significance of visual hallucinations and cerebral hypometabolism in the risk of dementia in Parkinson’s disease patients with mild cognitive impairment. Hum. Brain Mapp. 37 , 968–977 (2016).
Ramírez-Ruiz, B., Junqué, C., Martí, M.-J., Valldeoriola, F. & Tolosa, E. Neuropsychological deficits in Parkinson’s disease patients with visual hallucinations. Mov. Disord. 21 , 1483–1487 (2006).
Nagano-Saito, A. et al. Visual hallucination in Parkinson’s disease with FDG PET. Mov. Disord. 19 , 801–806 (2004).
Ramirez-Ruiz, B. et al. Brain response to complex visual stimuli in Parkinson’s patients with hallucinations: a functional magnetic resonance imaging study. Mov. Disord. 23 , 2335–2343 (2008).
Sanchez-Castaneda, C. et al. Frontal and associative visual areas related to visual hallucinations in dementia with Lewy bodies and Parkinson’s disease with dementia. Mov. Disord. 25 , 615–622 (2010).
Gama, R. L. et al. Structural brain abnormalities in patients with Parkinson’s disease with visual hallucinations: a comparative voxel-based analysis. Brain Cogn. 87 , 97–103 (2014).
Ibarretxe-Bilbao, N. et al. Hippocampal head atrophy predominance in Parkinson’s disease with hallucinations and with dementia. J. Neurol. 255 , 1324–1331 (2008).
Janzen, J. et al. The pedunculopontine nucleus is related to visual hallucinations in Parkinson’s disease: preliminary results of a voxel-based morphometry study. J. Neurol. 259 , 147–154 (2012).
Shine, J. M., Halliday, G. M., Naismith, S. L. & Lewis, S. J. G. Visual misperceptions and hallucinations in Parkinson’s disease: dysfunction of attentional control networks? Mov. Disord. 26 , 2154–2159 (2011).
Shine, J. M. et al. Imagine that: elevated sensory strength of mental imagery in individuals with Parkinson’s disease and visual hallucinations. Proc. Biol. Sci. 282 , 20142047 (2015).
Shine, J. M. et al. Abnormal connectivity between the default mode and the visual system underlies the manifestation of visual hallucinations in Parkinson’s disease: a task-based fMRI study. Parkinsons Dis. 1 , 15003 (2015).
Corbetta, M. & Shulman, G. L. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 3 , 201–215 (2002).
Fox, M. D. et al. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl Acad. Sci. USA 102 , 9673–9678 (2005).
Buckner, R. L. & DiNicola, L. M. The brain’s default network: updated anatomy, physiology and evolving insights. Nat. Rev. Neurosci. 20 , 593–608 (2019).
Lewis, G. J. & Bates, T. C. The long reach of the gene. Psychologist 26 , 194–198 (2013).
Google Scholar
Fox, M. D., Corbetta, M., Snyder, A. Z., Vincent, J. L. & Raichle, M. E. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc. Natl Acad. Sci. USA 103 , 10046–10051 (2006).
Eckert, M. A. et al. At the heart of the ventral attention system: the right anterior insula. Hum. Brain Mapp. 30 , 2530–2541 (2009).
Shine, J. M., Halliday, G. H., Carlos, M., Naismith, S. L. & Lewis, S. J. G. Investigating visual misperceptions in Parkinson’s disease: a novel behavioral paradigm. Mov. Disord. 27 , 500–505 (2012).
Hepp, D. H. et al. Loss of functional connectivity in patients with Parkinson disease and visual hallucinations. Radiology 285 , 896–903 (2017).
Article PubMed ADS Google Scholar
Miloserdov, K. et al. Aberrant functional connectivity of resting state networks related to misperceptions and intra-individual variability in Parkinson’s disease. Neuroimage Clin. 25 , 102076 (2020).
Dujardin, K. et al. What can we learn from fMRI capture of visual hallucinations in Parkinson’s disease? Brain Imaging Behav. 14 , 329–335 (2020).
Walpola, I. C. et al. Mind-wandering in Parkinson’s disease hallucinations reflects primary visual and default network coupling. Cortex 125 , 233–245 (2020).
Knolle, F. et al. Altered subcortical emotional salience processing differentiates Parkinson’s patients with and without psychotic symptoms. Neuroimage Clin. 27 , 10227 (2020).
Zarkali, A. et al. Changes in dynamic transitions between integrated and segregated states underlie visual hallucinations in Parkinson’s disease. Commun. Biol. 5 , 928 (2022).
Onofrj, M., Espay, A. J., Bonanni, L., Delli Pizzi, S. & Sensi, S. L. Hallucinations, somatic‐functional disorders of PD‐DLB as expressions of thalamic dysfunction. Mov. Disord. 34 , 1100–1111 (2019).
Zarkali, A. et al. Fiber-specific white matter reductions in Parkinson hallucinations and visual dysfunction. Neurology 94 , E1525–E1538 (2020).
Zarkali, A., McColgan, P., Leyland, L. A., Lees, A. J. & Weil, R. S. Longitudinal thalamic white and grey matter changes associated with visual hallucinations in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 93 , 169–179 (2022).
Zarkali, A. et al. Differences in network controllability and regional gene expression underlie hallucinations in Parkinson’s disease. Brain 143 , 3435–3448 (2021).
Thomas, G. E. C. et al. Changes in both top-down and bottom-up effective connectivity drive visual hallucinations in Parkinson’s disease. Brain Commun. 5 , fcac329 (2022).
Chaumon, M., Kveraga, K., Barrett, L. F. & Bar, M. Visual predictions in the orbitofrontal cortex rely on associative content. Cereb. Cortex 24 , 2899–2907 (2014).
Zarkali, A. et al. Increased weighting on prior knowledge in Lewy body-associated visual hallucinations. Brain Commun. 1 , fcz007 (2019).
Lefebvre, S. et al. Hallucinations and conscious access to visual inputs in Parkinson’s disease. Sci. Rep. 6 , 36284 (2016).
Perinelli, A., Tabarelli, D., Miniussi, C. & Ricci, L. Dependence of connectivity on geometric distance in brain networks. Sci. Rep. 9 , 13412 (2019).
Yao, N. et al. The default mode network is disrupted in parkinson’s disease with visual hallucinations. Hum. Brain Mapp. 35 , 5658–5666 (2014).
Franciotti, R. et al. Default mode network links to visual hallucinations: a comparison between Parkinson’s disease and multiple system atrophy. Mov. Disord. 30 , 1237–1247 (2015).
Yao, N. et al. Multimodal MRI of the hippocampus in Parkinson’s disease with visual hallucinations. Brain Struct. Funct. 221 , 287–300 (2016).
Yao, N. et al. Resting activity in visual and corticostriatal pathways in Parkinson’s disease with hallucinations. Parkinsonism Relat. Disord. 21 , 131–137 (2015).
Lee, J. Y. et al. Lateral geniculate atrophy in Parkinson’s with visual hallucination: a trans-synaptic degeneration? Mov. Disord. 31 , 547–554 (2016).
Miyata, M. et al. Optic radiation atrophy in Lewy body disease with visual hallucination on phase difference enhanced magnetic resonance images. Sci. Rep. 12 , 18556 (2022).
Hepp, D. H. et al. Damaged fiber tracts of the nucleus basalis of Meynert in Parkinson’s disease patients with visual hallucinations. Sci. Rep. 7 , 10112 (2017).
Yuki, N., Yoshioka, A., Mizuhara, R. & Kimura, T. Visual hallucinations and inferior longitudinal fasciculus in Parkinson’s disease. Brain Behav. 10 , e01883 (2020).
Zhong, J. M. et al. Why psychosis is frequently associated with Parkinson’s disease? Neural Regen. Res. 8 , 2548–2556 (2013).
PubMed PubMed Central ADS Google Scholar
Hall, J. M. et al. Changes in structural network topology correlate with severity of hallucinatory behavior in Parkinson’s disease. Netw. Neurosci. 3 , 521–538 (2019).
Rootes-Murdy, K., Goldsmith, D. R. & Turner, J. A. Clinical and structural differences in delusions across diagnoses: a systematic review. Front. Integr. Neurosci. 15 , 726321 (2022).
Factor, S. A., Molho, E. S., Podskalny, G. D. & Brown, D. Parkinson’s disease: drug-induced psychiatric states. Adv. Neurol. 65 , 115–138 (1995).
Goetz, C., Tanner, C. & Klawans, H. Pharmacology of hallucinations induced by long-term drug therapy. Am. J. Psychiatry 139 , 494–497 (1982).
de la Riva, P., Smith, K., Xie, S. X. & Weintraub, D. Course of psychiatric symptoms and global cognition in early Parkinson disease. Neurology 83 , 1096–1103 (2014).
Neumann, J. et al. Glucocerebrosidase mutations in clinical and pathologically proven Parkinson’s disease. Brain 132 , 1783–1794 (2009).
Dotchin, C. L., Jusabani, A. & Walker, R. W. Non-motor symptoms in a prevalent population with Parkinson’s disease in Tanzania. Parkinsonism Relat. Disord. 15 , 457–460 (2009).
Wolters, E. C. Dopaminomimetic psychosis in Parkinson’s disease patients: diagnosis and treatment. Neurology 52 , S10–S13 (1999).
McCutcheon, R. A. et al. Mesolimbic dopamine function is related to salience network connectivity: an integrative positron emission tomography and magnetic resonance study. Biol. Psychiatry 85 , 368–378 (2019).
Russo, M. et al. The pharmacology of visual hallucinations in synucleinopathies. Front. Pharmacol. 10 , 1379 (2019).
van der Zee, S. et al. Altered cholinergic innervation in de novo Parkinson’s disease with and without cognitive impairment. Mov. Disord. 37 , 713–723 (2022).
Bohnen, N. I. et al. Cholinergic system changes in Parkinson’s disease: emerging therapeutic approaches. Lancet Neurol. 21 , 381–392 (2022).
Whitehouse, P. J., Hedreen, J. C., White, C. L. & Price, D. L. Basal forebrain neurons in the dementia of Parkinson disease. Ann. Neurol. 13 , 243–248 (1983).
Perry, E. K. et al. Cholinergic correlates of cognitive impairment in Parkinson’s disease: comparisons with Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 48 , 413–421 (1985).
Shimada, H. et al. Mapping of brain acetylcholinesterase alterations in Lewy body disease by PET. Neurology 73 , 273–278 (2009).
Bohnen, N. I. et al. Progression of regional cortical cholinergic denervation in Parkinson’s disease. Brain Commun. 4 , fcac320 (2022).
Ray, N. J., Kanel, P. & Bohnen, N. I. Atrophy of the cholinergic basal forebrain can detect presynaptic cholinergic loss in Parkinson’s disease. Ann. Neurol. 93 , 991–998 (2023).
Manganelli, F. et al. Functional involvement of central cholinergic circuits and visual hallucinations in Parkinson’s disease. Brain 132 , 2350–2355 (2009).
Johnson, M. W., Hendricks, P. S., Barrett, F. S. & Griffiths, R. R. Classic psychedelics: an integrative review of epidemiology, therapeutics, mystical experience, and brain network function. Pharmacol. Ther. 197 , 83–102 (2019).
Hofmann, A. Psychotomimetic drugs; chemical and pharmacological aspects. Acta Physiol. Pharmacol. Neerl. 8 , 240–258 (1959).
Cheng, A. V. T. et al. Cortical serotonin-S2 receptor binding in Lewy body dementia, Alzheimer’s and Parkinson’s diseases. J. Neurol. Sci. 106 , 50–55 (1991).
Chen, C. et al. Post-synaptic 5-HT1A and 5-HT2A receptors are increased in Parkinson’s disease neocortex. Ann. N. Y. Acad. Sci. 861 , 288–289 (1998).
Article CAS PubMed ADS Google Scholar
Rasmussen, N. B. et al. 5-HT 2A receptor binding in the frontal cortex of Parkinson’s disease patients and alpha-synuclein overexpressing mice: a postmortem study. Parkinsons Dis. 2016 , 3682936 (2016).
Huot, P. et al. Increased 5-HT 2A receptors in the temporal cortex of parkinsonian patients with visual hallucinations. Mov. Disord. 25 , 1399–1408 (2010).
Ballanger, B. et al. Serotonin 2A receptors and visual hallucinations in Parkinson disease. Arch. Neurol. 67 , 416–421 (2010).
Burstein, E. S. Relevance of 5-HT2A receptor modulation of pyramidal cell excitability for dementia-related psychosis: implications for pharmacotherapy. CNS Drugs 35 , 727–741 (2021).
Papapetropoulos, S. Regional alpha-synuclein aggregation, dopaminergic dysregulation, and the development of drug-related visual hallucinations in Parkinson’s disease. J. Neuropsychiatry Clin. Neurosci. 18 , 149–157 (2006).
Barnes, N. M., Hales, T. G., Lummis, S. C. R. & Peters, J. A. The 5-HT3 receptor — the relationship between structure and function. Neuropharmacology 56 , 273–284 (2009).
Tsitsipa, E. et al. Selective 5HT3 antagonists and sensory processing: a systematic review. Neuropsychopharmacology 47 , 880–890 (2022).
Zhang, Z.-J. et al. Beneficial effects of ondansetron as an adjunct to haloperidol for chronic, treatment-resistant schizophrenia: a double-blind, randomized, placebo-controlled study. Schizophr. Res. 88 , 102–110 (2006).
Arnsten, A. F. T., Lin, C. H., Van Dyck, C. H. & Stanhope, K. J. The effects of 5-HT3 receptor antagonists on cognitive performance in aged monkeys. Neurobiol. Aging 18 , 21–28 (1997).
Gil-Bea, F. J. et al. Facilitation of cholinergic transmission by combined treatment of ondansetron with flumazenil after cortical cholinergic deafferentation. Neuropharmacology 47 , 225–232 (2004).
Garani, R., Watts, J. J. & Mizrahi, R. Endocannabinoid system in psychotic and mood disorders, a review of human studies. Prog. Neuropsychopharmacol. Biol. Psychiatry 106 , 110096 (2021).
Lu, H.-C. & Mackie, K. An introduction to the endogenous cannabinoid system. Biol. Psychiatry 79 , 516–525 (2016).
Katona, I. & Freund, T. F. Endocannabinoid signaling as a synaptic circuit breaker in neurological disease. Nat. Med. 14 , 923–930 (2008).
Marco, E. Endocannabinoid system and psychiatry: in search of a neurobiological basis for detrimental and potential therapeutic effects. Front. Behav. Neurosci. 5 , 63 (2011).
Van Laere, K. et al. Regional changes in type 1 cannabinoid receptor availability in Parkinson’s disease in vivo. Neurobiol. Aging 33 , 620.e1–620.e8 (2012).
Halff, E. F., Rutigliano, G., Garcia-Hidalgo, A. & Howes, O. D. Trace amine-associated receptor 1 (TAAR1) agonism as a new treatment strategy for schizophrenia and related disorders. Trends Neurosci. 46 , 60–74 (2023).
Dave, S., Weintraub, D., Aarsland, D. & ffytche, D. H. Drug and disease effects in Parkinson’s psychosis: revisiting the role of dopamine. Mov. Disord. Clin. Pract. 7 , 32–36 (2020).
Friedman, J. H. & Factor, S. A. Atypical antipsychotics in the treatment of drug-induced psychosis in Parkinson’s disease. Mov. Disord. 15 , 201–211 (2000).
Meco, G., Alessandria, A., Bonifati, V. & Giustini, P. Risperidone for hallucinations in levodopa-treated Parkinson’s disease patients. Lancet 343 , 1370–1371 (1994).
Goetz, C. G., Blasucci, L. M., Leurgans, S. & Pappert, E. J. Olanzapine and clozapine. Neurology 55 , 789–794 (2000).
Kashihara, K., Maeda, T. & Yoshida, K. Safety and tolerability of aripiprazole in patients with psychosis associated with Parkinson’s disease—results of a multicenter open trial. Neuropsychopharmacol. Rep. 42 , 135–141 (2022).
Younce, J. R., Davis, A. A. & Black, K. J. A systematic review and case series of ziprasidone for psychosis in Parkinson’s disease. J. Parkinsons Dis. 9 , 63–71 (2019).
Seppi, K. et al. Update on treatments for nonmotor symptoms of Parkinson’s disease—an evidence‐based medicine review. Mov. Disord. 34 , 180–198 (2019).
Weintraub, D. et al. Association of antipsychotic use with mortality risk in patients with Parkinson disease. JAMA Neurol. 73 , 535–541 (2016).
Pollak, P. et al. Clozapine in drug induced psychosis in Parkinson’s disease: a randomised, placebo controlled study with open follow up. J. Neurol. Neurosurg. Psychiatry 75 , 689–695 (2004).
Parkinson Study Group. Low-dose clozapine for the treatment of drug-induced psychosis in Parkinson’s disease. N. Engl. J. Med. 340 , 757–763 (1999).
Emre, M. et al. Rivastigmine for dementia associated with Parkinson’s disease. N. Engl. J. Med. 351 , 2509–2518 (2004).
Burn, D. et al. Effects of rivastigmine in patients with and without visual hallucinations in dementia associated wsith Parkinson’s disease. Mov. Disord. 21 , 1899–1907 (2006).
Article MathSciNet PubMed Google Scholar
Reading, P. J., Luce, A. K. & McKeith, I. G. Rivastigmine in the treatment of parkinsonian psychosis and cognitive impairment: preliminary findings from an open trial. Mov. Disord. 16 , 1171–1174 (2001).
Henderson, E. J. et al. Rivastigmine for gait stability in patients with Parkinson’s disease (ReSPonD): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol. 15 , 249–258 (2016).
d’Angremont, E., Begemann, M. J. H., van Laar, T. & Sommer, I. E. C. Cholinesterase inhibitors for treatment of psychotic symptoms in Alzheimer disease and Parkinson disease. JAMA Neurol. 80 , 813–823 (2023).
Vanover, K. E. et al. Pharmacological and behavioral profile of N -(4-fluorophenylmethyl)- N -(1-methylpiperidin-4-yl)- N -(4-(2-methylpropyloxy)phenylmethyl) carbamide (2 R ,3 R )-dihydroxybutanedioate (2:1) (ACP-103), a novel 5-hydroxytryptamine-2A receptor inverse agonist. J. Pharmacol. Exp. Ther. 317 , 910–918 (2006).
Cummings, J. et al. Pimavanserin for patients with Parkinson’s disease psychosis: a randomised, placebo-controlled phase 3 trial. Lancet 383 , 533–540 (2014).
Meltzer, H. Y. et al. Pimavanserin, a serotonin2A receptor inverse agonist, for the treatment of Parkinson’s disease psychosis. Neuropsychopharmacology 35 , 881–892 (2010).
Tariot, P. N. et al. Trial of pimavanserin in dementia-related psychosis. N. Engl. J. Med. 385 , 309–319 (2021).
Isaacson, S. H. et al. Efficacy results of pimavanserin from a multi-center, open-label extension study in Parkinson’s disease psychosis patients. Parkinsonism Relat. Disord. 87 , 25–31 (2021).
Ballard, C. G. et al. Long-term evaluation of open-label pimavanserin safety and tolerability in Parkinson’s disease psychosis. Parkinsonism Relat. Disord. 77 , 100–106 (2020).
Longardner, K. et al. Assessing the risks of treatment in Parkinson disease psychosis: an in-depth analysis. PLoS ONE 18 , e0278262 (2023).
Pham Nguyen, T. P., Thibault, D., Hamedani, A. G., Weintraub, D. & Willis, A. W. Atypical antipsychotic use and mortality risk in Parkinson disease. Parkinsonism Relat. Disord. 103 , 17–22 (2022).
Wildeboer, K. M., Zheng, L., Choo, K. S. & Stevens, K. E. Ondansetron results in improved auditory gating in DBA/2 mice through a cholinergic mechanism. Brain Res. 1300 , 41–50 (2009).
Hashimoto, K., Iyo, M., Freedman, R. & Stevens, K. E. Tropisetron improves deficient inhibitory auditory processing in DBA/2 mice: role of α7 nicotinic acetylcholine receptors. Psychopharmacology 183 , 13–19 (2005).
Kishi, T., Mukai, T., Matsuda, Y. & Iwata, N. Selective serotonin 3 receptor antagonist treatment for schizophrenia: meta-analysis and systematic review. Neuromolecular Med. 16 , 61–69 (2014).
Zoldan, J., Friedberg, G., Livneh, M. & Melamed, E. Psychosis in advanced Parkinson’s disease: treatment with ondansetron, a 5-HT3 receptor antagonist. Neurology 45 , 1305–1308 (1995).
Zoldan, J., Friedberg, G., Goldberg-Stern, H. & Melamed, E. Ondansetron for hallucinosis in advanced Parkinson’s disease. Lancet 341 , 562–563 (1993).
Kluger, B., Triolo, P., Jones, W. & Jankovic, J. The therapeutic potential of cannabinoids for movement disorders. Mov. Disord. 30 , 313–327 (2015).
Balash, Y. et al. Medical cannabis in Parkinson disease: real-life patients’ experience. Clin. Neuropharmacol. 40 , 268–272 (2017).
Chesney, E., Oliver, D. & McGuire, P. Cannabidiol (CBD) as a novel treatment in the early phases of psychosis. Psychopharmacology 239 , 1179–1190 (2022).
McGuire, P. et al. Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: a multicenter randomized controlled trial. Am. J. Psychiatry 175 , 225–231 (2018).
Zuardi, A. et al. Cannabidiol for the treatment of psychosis in Parkinson’s disease. J. Psychopharmacol. 23 , 979–983 (2009).
Chagas, M. H. N. et al. Effects of cannabidiol in the treatment of patients with Parkinson’s disease: an exploratory double-blind trial. J. Psychopharmacol. 28 , 1088–1098 (2014).
Koblan, K. S. et al. A non-D2-receptor-binding drug for the treatment of schizophrenia. N. Engl. J. Med. 382 , 1497–1506 (2020).
Isaacson, S. H. & Citrome, L. Hallucinations and delusions associated with Parkinson’s disease psychosis: safety of current treatments and future directions. Expert Opin. Drug Saf. 21 , 873–879 (2022).
Download references
Acknowledgements
This work was partially supported by funding from Centres de Recerca de Catalunya (CERCA) and Centro de Investigación Biomédica en Red, Enfermedades Neurodegenerativas (CIBERNED).
Author information
Authors and affiliations.
Movement Disorder Unit, Neurology Department, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain
Javier Pagonabarraga, Helena Bejr-Kasem, Saul Martinez-Horta & Jaime Kulisevsky
Department of Medicine, Autonomous University of Barcelona, Barcelona, Spain
Sant Pau Biomedical Research Institute (IIB-Sant Pau), Barcelona, Spain
Centro de Investigación en Red — Enfermedades Neurodegenerativas (CIBERNED), Madrid, Spain
You can also search for this author in PubMed Google Scholar
Contributions
J.P., H.B.-K. and S.M.-H. researched data for the article. All authors contributed substantially to discussion of the content, wrote the manuscript and reviewed and/or edited the manuscript before submission.
Corresponding author
Correspondence to Javier Pagonabarraga .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Peer review
Peer review information.
Nature Reviews Neurology thanks J. Friedman, S. Lewis and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Processing of information on the basis of incoming data from the environment to form a perception.
The belief that another person, often a friend or relative, has been replaced by an identical or near-identical impostor.
Illusions in which a real object is perceived as another entity, for example, a lamp in the living room is perceived as a standing person.
Quantification of the influence that a brain region has across the rest of the network.
Recurrent suspicions, without justification, regarding the fidelity of one’s spouse or sexual partner.
The experience that one’s feelings, impulses, thoughts or actions are not one’s own but are being imposed by some external force.
The false belief that innocuous events or mere coincidences have strong personal significance.
The false belief that someone is stealing one’s belongings.
A visual illusion in which the shape of an object appears distorted.
The influence that a node exerts over another under a network model of causal dynamics, which defines the mechanisms of neuronal coupling.
Neuronal networks subserving the processing of internal mentations independently from environmental stimuli.
Neuronal networks and engrams involved in directing mental processes towards environmental stimuli.
The belief that another person, often a friend or relative, is able to disguise themself as an unfamiliar person to influence the behaviour of the patient.
Functional interactions among different brain regions.
The belief that another person, often a friend or relative, has been transformed both physically and psychologically into another person.
A visual illusion in which stationary objects seem to be moving.
A visual illusion in which colours of an object appear different from those in reality.
Misidentification and reduplication of oneself in the mirror.
Visual illusions in which formless visual stimuli, such as clouds, tree bark or patterns in carpets or wallpaper, are perceived as human faces or animals.
Pervasive distrust and suspicion of others such that their motives are interpreted as malevolent (exploiting, harming, threatening or deceiving).
The belief that a double of another person exists. Also known as the syndrome of subjective doubles.
The belief that oneself has been relocated to an identical or near-identical duplicated place.
Time delay between a perception and movements previously associated with that perception.
Visual perceptions of an animate being, object or event in the absence of any external stimulus.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Cite this article.
Pagonabarraga, J., Bejr-Kasem, H., Martinez-Horta, S. et al. Parkinson disease psychosis: from phenomenology to neurobiological mechanisms. Nat Rev Neurol 20 , 135–150 (2024). https://doi.org/10.1038/s41582-023-00918-8
Download citation
Accepted : 13 December 2023
Published : 15 January 2024
Issue Date : March 2024
DOI : https://doi.org/10.1038/s41582-023-00918-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Mind-wandering in Parkinson's disease hallucinations reflects primary visual and default network coupling
Research output : Contribution to journal › Article › peer-review
Visual hallucinations are an underappreciated symptom affecting the majority of patients during the natural history of Parkinson's disease. Little is known about other forms of abstract and internally generated cognition – such as mind-wandering – in this population, but emerging evidence suggests that an interplay between the brain's primary visual and default networks might play a crucial role in both internally generated imagery and hallucinations. Here, we explored the association between mind-wandering and visual hallucinations in Parkinson's disease, and their relationship with brain network coupling. We administered a validated thought-sampling task to 38 Parkinson's disease patients (18 with hallucinations; 20 without) and 40 controls, to test the hypothesis that individuals with hallucinations experience an increased frequency of mind-wandering. Group differences in the association between mind-wandering frequency and brain network coupling were also examined using resting state functional magnetic resonance imaging. Our results showed that patients with hallucinations exhibited significantly higher mind-wandering frequencies compared to non-hallucinators, who in turn had reduced levels of mind-wandering relative to controls. At the level of brain networks, inter-network connectivity and seed-to-voxel analyses identified that increased mind-wandering in the hallucinating versus non-hallucinating group was associated with greater coupling between the primary visual cortex and dorsal default network. Taken together, our results suggest a relative preservation of mind-wandering in Parkinson's disease patients who experience visual hallucinations, which is associated with increased visual cortex-default network coupling. We propose that the preservation of florid abstract and internally generated cognition in the context of the Parkinson's disease can contribute to visual hallucinations, whereas healthy individuals experience only the vivid images of the mind's eye. These findings refine current models of visual hallucinations by identifying a specific cognitive phenomenon and neural substrate consistent with the top-down influences over perception that have been implicated in hallucinations across neuropsychiatric disorders.
- Default network
- Mind-wandering
- Parkinson's disease
- Resting state functional magnetic resonance imaging
- Visual hallucinations
ASJC Scopus subject areas
- Neuropsychology and Physiological Psychology
- Experimental and Cognitive Psychology
- Cognitive Neuroscience
Access to Document
- 10.1016/j.cortex.2019.12.023
Other files and links
- Link to publication in Scopus
Fingerprint
- Hallucinations Medicine & Life Sciences 100%
- Parkinson Disease Medicine & Life Sciences 73%
- brain Social Sciences 42%
- cognition Social Sciences 20%
- Visual Cortex Medicine & Life Sciences 18%
- Brain Medicine & Life Sciences 17%
- Cognition Medicine & Life Sciences 13%
- experience Social Sciences 11%
T1 - Mind-wandering in Parkinson's disease hallucinations reflects primary visual and default network coupling
AU - Walpola, Ishan C.
AU - Muller, Alana J.
AU - Hall, Julie M.
AU - Andrews-Hanna, Jessica R.
AU - Irish, Muireann
AU - Lewis, Simon J.G.
AU - Shine, James M.
AU - O'Callaghan, Claire
N1 - Funding Information: We thank Kelly Diederen for providing valuable comments on the manuscript. AJM is supported by an Australian Postgraduate Award through the University of Sydney. JMH is supported by a Western Sydney University Postgraduate Award. JAH is supported by a National Institutes of Aging Arizona Alzheimer's Disease Core Center grant (P30 AG019610). MI is supported by an Australian Research Council Future Fellowship (FT160100096) and an Australian Research Council Discovery Project (DP180101548). SJGL is supported by an NHMRC-ARC Dementia Fellowship (#1110414). JMS is supported by a National Health and Medical Research Council CJ Martin Fellowship (1072403). CO is supported by a National Health and Medical Research Council Neil Hamilton Fairley Fellowship (1091310) and by the Wellcome Trust (200181/Z/15/Z). The study was supported by a Seed Grant from Parkinson's NSW and funding to Forefront, a collaborative research group dedicated to the study of non-Alzheimer disease degenerative dementias, from the National Health and Medical Research Council of Australia program grant (#1037746 and #1095127). Funding Information: We thank Kelly Diederen for providing valuable comments on the manuscript. AJM is supported by an Australian Postgraduate Award through the University of Sydney . JMH is supported by a Western Sydney University Postgraduate Award . JAH is supported by a National Institutes of Aging Arizona Alzheimer’s Disease Core Center grant ( P30 AG019610 ). MI is supported by an Australian Research Council Future Fellowship ( FT160100096 ) and an Australian Research Council Discovery Project ( DP180101548 ). SJGL is supported by an NHMRC-ARC Dementia Fellowship ( #1110414 ). JMS is supported by a National Health and Medical Research Council CJ Martin Fellowship ( 1072403 ). CO is supported by a National Health and Medical Research Council Neil Hamilton Fairley Fellowship ( 1091310 ) and by the Wellcome Trust ( 200181/Z/15/Z ). The study was supported by a Seed Grant from Parkinson's NSW and funding to Forefront, a collaborative research group dedicated to the study of non-Alzheimer disease degenerative dementias, from the National Health and Medical Research Council of Australia program grant (# 1037746 and # 1095127 ). Publisher Copyright: © 2020 Elsevier Ltd
PY - 2020/4
Y1 - 2020/4
N2 - Visual hallucinations are an underappreciated symptom affecting the majority of patients during the natural history of Parkinson's disease. Little is known about other forms of abstract and internally generated cognition – such as mind-wandering – in this population, but emerging evidence suggests that an interplay between the brain's primary visual and default networks might play a crucial role in both internally generated imagery and hallucinations. Here, we explored the association between mind-wandering and visual hallucinations in Parkinson's disease, and their relationship with brain network coupling. We administered a validated thought-sampling task to 38 Parkinson's disease patients (18 with hallucinations; 20 without) and 40 controls, to test the hypothesis that individuals with hallucinations experience an increased frequency of mind-wandering. Group differences in the association between mind-wandering frequency and brain network coupling were also examined using resting state functional magnetic resonance imaging. Our results showed that patients with hallucinations exhibited significantly higher mind-wandering frequencies compared to non-hallucinators, who in turn had reduced levels of mind-wandering relative to controls. At the level of brain networks, inter-network connectivity and seed-to-voxel analyses identified that increased mind-wandering in the hallucinating versus non-hallucinating group was associated with greater coupling between the primary visual cortex and dorsal default network. Taken together, our results suggest a relative preservation of mind-wandering in Parkinson's disease patients who experience visual hallucinations, which is associated with increased visual cortex-default network coupling. We propose that the preservation of florid abstract and internally generated cognition in the context of the Parkinson's disease can contribute to visual hallucinations, whereas healthy individuals experience only the vivid images of the mind's eye. These findings refine current models of visual hallucinations by identifying a specific cognitive phenomenon and neural substrate consistent with the top-down influences over perception that have been implicated in hallucinations across neuropsychiatric disorders.
AB - Visual hallucinations are an underappreciated symptom affecting the majority of patients during the natural history of Parkinson's disease. Little is known about other forms of abstract and internally generated cognition – such as mind-wandering – in this population, but emerging evidence suggests that an interplay between the brain's primary visual and default networks might play a crucial role in both internally generated imagery and hallucinations. Here, we explored the association between mind-wandering and visual hallucinations in Parkinson's disease, and their relationship with brain network coupling. We administered a validated thought-sampling task to 38 Parkinson's disease patients (18 with hallucinations; 20 without) and 40 controls, to test the hypothesis that individuals with hallucinations experience an increased frequency of mind-wandering. Group differences in the association between mind-wandering frequency and brain network coupling were also examined using resting state functional magnetic resonance imaging. Our results showed that patients with hallucinations exhibited significantly higher mind-wandering frequencies compared to non-hallucinators, who in turn had reduced levels of mind-wandering relative to controls. At the level of brain networks, inter-network connectivity and seed-to-voxel analyses identified that increased mind-wandering in the hallucinating versus non-hallucinating group was associated with greater coupling between the primary visual cortex and dorsal default network. Taken together, our results suggest a relative preservation of mind-wandering in Parkinson's disease patients who experience visual hallucinations, which is associated with increased visual cortex-default network coupling. We propose that the preservation of florid abstract and internally generated cognition in the context of the Parkinson's disease can contribute to visual hallucinations, whereas healthy individuals experience only the vivid images of the mind's eye. These findings refine current models of visual hallucinations by identifying a specific cognitive phenomenon and neural substrate consistent with the top-down influences over perception that have been implicated in hallucinations across neuropsychiatric disorders.
KW - Default network
KW - Mind-wandering
KW - Parkinson's disease
KW - Resting state functional magnetic resonance imaging
KW - Visual hallucinations
UR - http://www.scopus.com/inward/record.url?scp=85079155081&partnerID=8YFLogxK
UR - http://www.scopus.com/inward/citedby.url?scp=85079155081&partnerID=8YFLogxK
U2 - 10.1016/j.cortex.2019.12.023
DO - 10.1016/j.cortex.2019.12.023
M3 - Article
C2 - 32058090
AN - SCOPUS:85079155081
SN - 0010-9452
JO - Cortex
JF - Cortex
Cognitive Changes

Some people with Parkinson’s disease (PD) experience mild cognitive impairment. Feelings of distraction or disorganization can accompany cognitive impairment, along with finding it difficult to plan and accomplish tasks.
It may be harder to focus in situations that divide your attention, like a group conversation. When facing a task or situation on their own, a person with PD may feel overwhelmed by having to make choices. They may also have difficulty remembering information or have trouble finding the right words when speaking. These changes can range from being annoying to interfering with managing household affairs.
To some degree, cognitive impairment affects many people with PD. The same brain changes that lead to motor symptoms can also result in slowness in memory and thinking. Stress, medication and depression can also contribute to these changes.
Symptoms of mild cognitive impairment (MCI) often do not interfere with home and work life. They may not even be noticeable, but can be detected through testing. Doctors used to believe that cognitive changes did not develop until middle to late-stage PD , but recent research suggests that mild changes may be present at the time of diagnosis.
Tell your doctor if you have concerns about cognitive changes. You may need to change your medication or see a neurologist or neuropsychologist for assessment. An occupational therapist can also help you find strategies for adapting and coping with these symptoms. A speech therapist can help with language difficulties.
In general, mental and motor decline tend to occur together as the disease progresses. Significant cognitive impairment in PD is often associated with:
- Caregiver distress
- Worse day-to-day function
- Diminished quality of life
- Poorer treatment outcomes
- Greater medical costs due to nursing home placements
- Increased mortality
Cognitive impairment is different from dementia, which is when cognitive impairments occur in more than one area of cognition, leading to more severe loss of intellectual abilities that interferes with daily, independent living. While 20% to 50% of people with PD will experience mild cognitive impairment, not all lead to a dementia diagnosis.
Two long-term studies suggest that many people with PD will eventually develop a mild form of dementia as the disease progresses, usually many years after their initial diagnosis. One medication, Exelon (rivastigmine tartrate), can treat dementia in PD. Other medications are being studied.
What causes cognitive changes in people with PD?
One cause is a drop in the level of dopamine, the neurotransmitter that is involved in regulating the body’s movements. However, the cognitive changes associated with dopamine declines are typically mild and restricted.
Other brain changes are likely also involved in cognitive decline in PD. Scientists are looking at changes in two other chemical messengers — acetylcholine and norepinephrine — as possible additional causes of memory and executive function loss in Parkinson’s.
Effects of Cognitive Changes
The cognitive changes that accompany Parkinson’s early on tend to be limited to one or two mental areas, with severity varying from person to person. Areas most often affected include:
- Difficulty with complex tasks that require person with PD to maintain or shift their attention.
- Problems with mental calculations or concentrating during a task.
Speed of Mental Processing
- Slowing in thinking is often associated with depression in PD.
- Signs include: a delay in responding to verbal or behavioral stimuli, taking longer to complete tasks and difficulty retrieving information from memory.
Problem-solving or Executive Function
- Trouble planning and completing activities.
- Difficulties in generating, maintaining, shifting and blending different ideas and concepts.
- More concrete in approach to tasks.
- Loved ones can help the person with PD by providing cues, reminders and greater structure of activity.
Memory Issues
- The basal ganglia and frontal lobes of the brain (both help the brain organize and recall of information) may be damaged in PD.
- Difficulty with common tasks such as making coffee, balancing a checkbook, etc.
- People with dementia can experience both short-term and long-term memory impairment.
Language Abnormalities
- Issues with word-finding, known as “tip of the tongue” phenomenon.
- Difficulty with language when under pressure or stress.
- Difficulty comprehending complex sentences where the question or information is included with other details.
- Problems with production of language and dysarthria — slurred or unarticulated speech due to weakened muscles caused by brain changes.
- Problems in naming or misnaming objects — more common in middle to late stages of PD.
Visuospatial Difficulties
- During early PD stages: difficulty with measuring distance and depth perception, which may interfere with parking a car or remembering where the car is parked.
- During advanced PD: in combination with dementia, problems with processing information about their surroundings or environment.
- Subtle visual-perceptual problems may contribute to the visual misperceptions or illusions.
- Increased chances of visual misperceptions or illusions in low-light situations (like nighttime) and if experiencing other visual problems (like macular degeneration).
- In severe cases, problems telling apart non-familiar faces or recognizing emotional expressions.
How are cognitive issues diagnosed?
Common ways to assess and diagnose cognitive disorders:
- Interview the person with PD.
- Ask family members or care partners about their observations.
- Administer cognitive screening tests such as the Mini-Mental State Examination (MMSE) or Montreal Cognitive Assessment (MOCA). The neurologist will ask questions that evaluate the person’s understanding of where and who they are, the date and year, attention, memory, language and problem-solving skills.
- A neurologist may suggest seeing a clinical neuropsychologist for a more detailed assessment.
- Neuropsychological assessment can be an important diagnostic tool for differentiating PD from other illnesses such as Alzheimer's disease, stroke or dementia.
How are cognitive changes in PD different than Alzheimer’s disease?
Overall, dementia produces a greater impact on social and occupational functioning in PD than Alzheimer’s due to the combination of motor and cognitive impairments.
There is some overlap between symptoms and biological changes seen in Alzheimer’s and PD. However, it is less likely for both disorders to occur at the same time. Development of dementia in people with PD represents progression of the disease, usually after several years of motor impairment.
Dementia may or may not occur in people with PD. According to recent research, 30% of people with Parkinson’s do not develop dementia as part of the disease progression.
See 10 Signs of Alzheimer’s.
What co-existing conditions affect thinking and memory?
There are other factors that can have a negative impact on a person’s cognitive skills, such as disorders of mood, anxiety and sleep. In some cases, these factors can make memory and thinking deficits worse, as well as directly affect a person’s quality of life.
- Up to 50% of people with PD experience some form of depression during the disease.
- More likely to occur in people who experience severe cognitive impairment.
- Successful treatment of depression with medication and psychotherapy can improve cognitive symptoms.
- Can make it difficult to control motor symptoms (such as tremor and balance problems) in PD.
- Tends to be more severe in people with worse motor symptoms.
- May be as common as depression in Parkinson’s.
- While less studied, up to 40% of people with PD experience some form of anxiety.
- Can interfere with memory storage, disrupt attention and complex task performance. For example, most people remember going blank on a school exam when feeling anxious.
- Negatively impacts social life. People with poorly controlled anxiety often avoid social situations, which can impact family and work relationships.
- People with PD may experience anticipatory anxiety in situations where they have to use cognitive skills.
- Similar to depression, successful treatment can lead to improvement of cognitive problems related to anxiety.
Sleep Issues
- The impact of poor sleep on attention, alertness and memory are well-known.
- Problems with falling and staying asleep are common in PD, especially as the disease progresses.
- Mild reductions in sleep can directly impair attention, judgment and the ability to multi-task because people with PD have a lower cognitive reserve or resistance of the brain to stressors.
- Undergoing a sleep study examines sleeping patterns and how often sleep is disrupted.
- Sleep problems are often addressed with medication and behavioral treatments. As sleep improves, its impact on thinking and memory is reduced.
Four types of sleep problems have been reported in PD:
- Issues staying asleep and early morning awakening (insomnia).
- Involuntary movements and pain that interrupt sleep.
- Increased nighttime urination.
- Nighttime agitation, vivid dreams and visual misperceptions or hallucinations.
- Just as fatigue can cause problems with movement and walking in PD, it can also impair thinking and memory. For example, a person with PD may have difficulty performing a complex cognitive task (like working on taxes over extended periods).
- Maximize attention and energy resources by dividing tasks into more manageable 10 to 15-minute sections. This helps minimize fatigue and keep you on task.
- Be aware that as the day wears on, people with PD may begin to fatigue — physically and cognitively.
- Medications can help improve energy and alertness (methylphenidate (Ritalin®) and modafinil (Provigil®)), but many have yet to be studied extensively for PD and fatigue.
Some medications used to treat PD have also been shown to have stimulating effects on thinking and energy levels (like selegiline (Eldepryl®) and amantadine).
Seeking Help for Cognitive Changes
Cognitive change is a sensitive issue. In fact, the doctor is often as hesitant to address this subject as the person with PD is to ask about it. Sometimes, the doctor will delay discussing cognitive impairment out of concern for the person who is still coping with the shock of a new PD diagnosis or struggling with motor symptoms.
For this reason, the person with PD often needs to be the one to initiate the conversation. Tell your doctor if you or your loved one is experiencing problems that upset the family or cause interruptions at work.
Cognitive issues are never too mild to address with your care team. A doctor can provide ways to help, often referring you to a psychiatrist, neuropsychologist, speech or occupational therapist for further evaluation and assistance. The neuropsychological evaluation can be particularly useful, especially in the early stages of a cognitive problem. Having this baseline test can help the doctor determine whether future changes are related to medications, the progression of the PD itself or to other factors such as depression.
When reporting symptoms of mild cognitive impairment, the doctor will first want to rule out causes other than PD, such as vitamin B-12 deficiency, depression, fatigue or sleep disturbances. It should be noted that PD does not cause sudden changes in mental functioning. If a sudden change occurs, the cause is likely to be something else, such as a medication side-effect.
If cognitive symptoms are traceable to PD, there are drug therapies available. Though developed for Alzheimer’s, these medications have been found to have some effect in PD. These include rivastigmine (the only medication approved by the FDA for dementia in PD), donepezil and galantamine. In addition, a person with attention difficulties that are due to daytime sleepiness may benefit from stimulants.
How are cognitive problems treated?
Much remains to be learned about the basic biology that underlies cognitive changes in PD. Researchers work towards the development of diagnostic tests to identify people who seem to be at greatest risk for cognitive changes and to differentiate cognitive problems in people with PD from those that occur in another disorder — related but different — known as dementia with Lewy bodies. A combination of medications and behavioral strategies is usually the best treatment for cognitive problems in PD.
Cognitive Remediation Therapy
For those with milder cognitive deficits, cognitive remediation therapy is a treatment that emphasizes teaching alternative ways to compensate for memory or thinking problems. In this treatment, the clinician uses information from neuropsychological testing to identify cognitive strengths that can be used to help overcome weaker areas of thinking.
- While widely used in the treatment of cognitive problems resulting from brain injury or stroke, there has been less use of this technique in people with PD.
- Does not reverse or cure cognitive disorders, but instead teaches strategies that can help with daily functioning and coping with cognitive problems.
- Depending on the severity of cognitive impairment, many can use these skills independently.
- In cases where the person is more impaired, care partners or family members can help apply these strategies.
- Usually conducted by a neuropsychologist or speech-language pathologist, who is specially trained in these techniques and can provide a supportive environment for the person with PD to express concerns and frustrations over changes in mental functioning.
- Works best with milder forms of cognitive deficits, as it requires insight into the person’s own memory and thinking problems.
Behavioral Management
In this type of treatment, changes in the environment can be made to help minimize memory, visual-perceptual or orientation difficulties.
- Strategies include simplifying the décor of the living area to reduce excessive stimuli and minimize confusion and using a nightlight or low-level lighting to reduce visual misperceptions and confusion at nighttime.
- Behavioral strategies can help deal with other problems such as impulsivity, wandering, poor initiation and problems with communication.
- A person with PD may benefit from a regular routine in their day-to-day activities and feel more comfortable with a clear, structured schedule.
Tips for Care Partners
- Offer help only when asked.
- Prompt the person — for example, instead of asking, “Did anyone call?” ask, “Did Linda call?”
- Say the name of the person and make eye contact when speaking to gain and hold attention.
- Put reminder notes and lists in a prominent place.
- Keep things in routine places.
- To ensure medications are taken on time, provide a dispenser, perhaps with a built-in alarm.
- Use photos on cell phone contact entries to prompt face-name association.
- If the person is searching for a word, provide a cue, such as, “the word you are looking for probably begins with ‘d’.”
- Do not finish the sentences of a person who needs more time to put them together.
- When presenting the person with a list of actions, first verbalize them, then write them down.
- Ask questions to moderate the conversation pace and allow catch up and reinforcement.
Page reviewed by Dr. Kathryn P Moore, Movement Disorders neurologist at Duke Health, a Parkinson's Foundation Center of Excellence.
Related Materials
Cognition: a mind guide to parkinson's disease, episode 27: more than movement: addressing cognitive and behavioral challenges in caring for pd, episode 65: recognizing non-motor symptoms in pd, related blog posts.

Mental Health Tips for Cognition, Mood and Sleep

Care Partner Deep Dive: Three Experts Discuss Sleep, Cognition and Mood in Parkinson's

Tips from the Pros: Maintaining Cognitive Brain Health in Parkinson's Disease
Parkinson's connection, personal information.
- Virtual Tour
- Ask the Brain
- Message from the Director
- The McGoverns
- Administration
- Explore the Brain
- Polina Anikeeva
- Emilio Bizzi
- Martha Constantine-Paton
- Robert Desimone
- James DiCarlo
- Ev Fedorenko
- Michale Fee
- Guoping Feng
- John Gabrieli
- Ann Graybiel
- Mark Harnett
- H. Robert Horvitz
- Alan Jasanoff
- Mehrdad Jazayeri
- Nancy Kanwisher
- Josh McDermott
- Tomaso Poggio
- Rebecca Saxe
- Nidhi Seethapathi
- Guangyu Robert Yang
- Satrajit Ghosh
- Dimitrios Pantazis
- Ian Wickersham
- Brain Imaging
- Cellular & Molecular Neuroscience
- Cognitive Neuroscience
- Computational Neuroscience
- Genome Engineering
- Neurotechnology
- Systems Neuroscience
- Alzheimer’s Disease
- Autism Spectrum Disorder
- Bipolar Disorder
- Huntington’s Disease
- Obsessive-Compulsive Disorder
- Parkinson’s Disease
- Schizophrenia
- Participate in a Study
- Community Resources
- Athinoula A. Martinos Imaging Center
- Poitras Center for Psychiatric Disorders Research
- Yang Tan Collective
- Join Our Mailing List
- Newsletter Archive
- Sponsored Researchers
- Meet Our Supporters
- Events Calendar
- McGovern Institute Annual Symposium
- Edward M. Scolnick Prize in Neuroscience
- Phillip A. Sharp Lecture in Neural Circuits
Dynamic brain activity associated with mind wandering, revealing possible new targets for brain disorders.

Image: Hannah Moore

What’s happening in your brain when you’re spacing out?
by Eva Botkin-Kowacki | March 25, 2021 May 24, 2023
Categories: Brain Imaging , ADHD , Anxiety , Depression , John Gabrieli , Martinos Imaging Center , Poitras Center for Psychiatric Disorders Research
This story is adapted from a News@Northeastern post .
We all do it. One second you’re fully focused on the task in front of you, a conversation with a friend, or a professor’s lecture, and the next second your mind is wandering to your dinner plans.
But how does that happen?
“We spend so much of our daily lives engaged in things that are completely unrelated to what’s in front of us,” says Aaron Kucyi, neuroscientist and principal research scientist in the department of psychology at Northeastern. “And we know very little about how it works in the brain.”
So Kucyi and colleagues at Massachusetts General Hospital, Boston University, and the McGovern Institute at MIT started scanning people’s brains using functional magnetic resonance imaging (fMRI) to get an inside look. Their results, published Friday in the journal Nature Communications , add complexity to our understanding of the wandering mind.
It turns out that spacing out might not deserve the bad reputation that it receives. Many more parts of the brain seem to be engaged in mind-wandering than previously thought, supporting the idea that it’s actually a quite dynamic and fundamental function of our psychology.
“Many of those things that we do when we’re spacing out are very adaptive and important to our lives,” says Kucyi, the paper’s first author. We might be drafting an email in our heads while in the shower, or trying to remember the host’s spouse’s name while getting dressed for a party. Moments when our minds wander can allow space for creativity and planning for the future, he says, so it makes sense that many parts of the brain would be engaged in that kind of thinking.
But mind wandering may also be detrimental, especially for those suffering from mental illness, explains the study’s senior author, Susan Whitfield-Gabrieli. “For many of us, mind wandering may be a healthy, positive and constructive experience, like reminiscing about the past, planning for the future, or engaging in creative thinking,” says Whitfield-Gabrieli, a professor of psychology at Northeastern University and a McGovern Institute research affiliate. “But for those suffering from mental illness such as depression , anxiety or psychosis, reminiscing about the past may transform into ruminating about the past, planning for the future may become obsessively worrying about the future and creative thinking may evolve into delusional thinking.”
Identifying the brain circuits associated with mind wandering, she says, may reveal new targets and better treatment options for people suffering from these disorders.
Inside the wandering mind
To study wandering minds, the researchers first had to set up a situation in which people were likely to lose focus. They recruited test subjects at the McGovern Institute’s Martinos Imaging Center to complete a simple, repetitive, and rather boring task. With an fMRI scanner mapping their brain activity, participants were instructed to press a button whenever an image of a city scene appeared on a screen in front of them and withhold a response when a mountain image appeared.
Throughout the experiment, the subjects were asked whether they were focused on the task at hand. If a subject said their mind was wandering, the researchers took a close look at their brain scans from right before they reported loss of focus. The data was then fed into a machine-learning algorithm to identify patterns in the neurological connections involved in mind-wandering (called “stimulus-independent, task-unrelated thought” by the scientists).
Scientists previously identified a specialized system in the brain considered to be responsible for mind-wandering. Called the “default mode network,” these parts of the brain activated when someone’s thoughts were drifting away from their immediate surroundings and deactivated when they were focused. The other parts of the brain, that theory went, were quiet when the mind was wandering, says Kucyi.
The “default mode network” did light up in Kucyi’s data. But parts of the brain associated with other functions also appeared to activate when his subjects reported that their minds had wandered.
For example, the “default mode network” and networks in the brain related to controlling or maintaining a train of thought also seemed to be communicating with one another, perhaps helping explain the ability to go down a rabbit hole in your mind when you’re distracted from a task. There was also a noticeable lack of communication between the “default mode network” and the systems associated with sensory input, which makes sense, as the mind is wandering away from the person’s immediate environment.
“It makes sense that virtually the whole brain is involved,” Kucyi says. “Mind-wandering is a very complex operation in the brain and involves drawing from our memory, making predictions about the future, dynamically switching between topics that we’re thinking about, fluctuations in our mood, and engaging in vivid visual imagery while ignoring immediate visual input,” just to name a few functions.
The “default mode network” still seems to be key, Kucyi says. Virtual computer analysis suggests that if you took the regions of the brain in that network out of the equation, the other brain regions would not be able to pick up the slack in mind-wandering.
Kucyi, however, didn’t just want to identify regions of the brain that lit up when someone said their mind was wandering. He also wanted to be able to use that generalized pattern of brain activity to be able to predict whether or not a subject would say that their focus had drifted away from the task in front of them.
That’s where the machine-learning analysis of the data came in. The idea, Kucyi says, is that “you could bring a new person into the scanner and not even ask them whether they were mind-wandering or not, and have a good estimate from their brain data whether they were.”
The ADHD brain
To test the patterns identified through machine learning, the researchers brought in a new set of test subjects – people diagnosed with ADHD . When the fMRI scans lit up the parts of the brain Kucyi and his colleagues had identified as engaged in mind-wandering in the first part of the study, the new test subjects reported that their thoughts had drifted from the images of cities and mountains in front of them. It worked.
Kucyi doesn’t expect fMRI scans to become a new way to diagnose ADHD, however. That wasn’t the goal. Perhaps down the road it could be used to help develop treatments, he suggests. But this study was focused on “informing the biological mechanisms behind it.”
John Gabrieli , a co-author on the study and director of the imaging center at MIT’s McGovern Institute, adds that “there is recent evidence that ADHD patients with more mind-wandering have many more everyday practical and clinical difficulties than ADHD patients with less mind-wandering. This is the first evidence about the brain basis for that important difference, and points to what neural systems ought to be the targets of intervention to help ADHD patients who struggle the most.”
For Kucyi, the study of “mind-wandering” goes beyond ADHD. And the contents of those straying thoughts may be telling, he says.
“We just asked people whether they were focused on the task or away from the task, but we have no idea what they were thinking about,” he says. “What are people thinking about? For example, are those more positive thoughts or negative thoughts?” Such answers, which he hopes to explore in future research, could help scientists better understand other pathologies such as depression and anxiety, which often involve rumination on upsetting or worrisome thoughts.
Whitfield-Gabrieli and her team are already exploring whether behavioral interventions, such as mindfulness based real-time fMRI neurofeedback, can be used to help train people suffering from mental illness to modulate their own brain networks and reduce hallucinations, ruminations, and other troubling symptoms.
“We hope that our research will have clinical implications that extend far beyond the potential for identifying treatment targets for ADHD,” she says.
Paper: "Prediction of stimulus-independent and task-unrelated thought from functional brain networks"
Using MRI, engineers have found a way to detect light deep in the brain
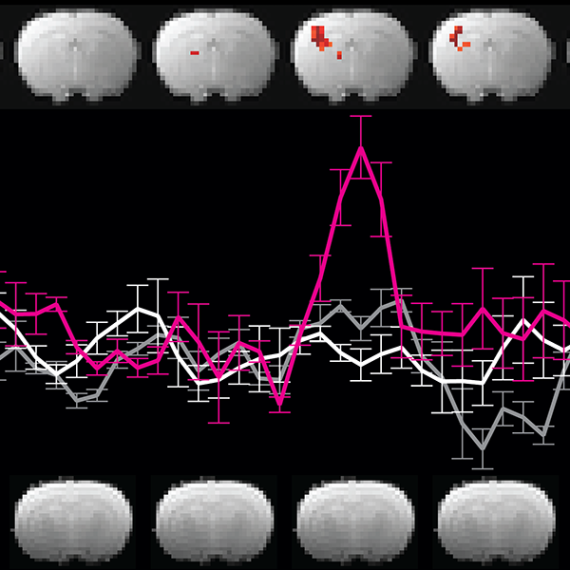
Reevaluating an approach to functional brain imaging
For people who speak many languages, there’s something special about their native tongue
Advertisement
Reconceptualizing mind wandering from a switching perspective
- Open access
- Published: 29 March 2022
- Volume 87 , pages 357–372, ( 2023 )
Cite this article
You have full access to this open access article

- Yi-Sheng Wong ORCID: orcid.org/0000-0002-5752-4679 1 , 2 , 3 ,
- Adrian R. Willoughby ORCID: orcid.org/0000-0002-4214-2635 3 , 4 &
- Liana Machado ORCID: orcid.org/0000-0002-0856-3831 1 , 2
6853 Accesses
7 Citations
21 Altmetric
Explore all metrics
Mind wandering is a universal phenomenon in which our attention shifts away from the task at hand toward task - unrelated thoughts. Despite it inherently involving a shift in mental set, little is known about the role of cognitive flexibility in mind wandering. In this article we consider the potential of cognitive flexibility as a mechanism for mediating and/or regulating the occurrence of mind wandering. Our review begins with a brief introduction to the prominent theories of mind wandering—the executive failure hypothesis, the decoupling hypothesis, the process - occurrence framework, and the resource - control account of sustained attention. Then, after discussing their respective merits and weaknesses, we put forward a new perspective of mind wandering focused on cognitive flexibility, which provides an account more in line with the data to date, including why older populations experience a reduction in mind wandering. After summarizing initial evidence prompting this new perspective, drawn from several mind - wandering and task - switching studies, we recommend avenues for future research aimed at further understanding the importance of cognitive flexibility in mind wandering.
Similar content being viewed by others

Self-reported mind wandering reflects executive control and selective attention

The Current State of Mind: a Systematic Review of the Relationship Between Mindfulness and Mind-Wandering
The metronome response task for measuring mind wandering: Replication attempt and extension of three studies by Seli et al
Avoid common mistakes on your manuscript.
Introduction
In the past two decades, there has been growing interest in understanding the basic psychological processes of mind wandering and its underlying mechanisms (for a review, see Kvavilashvili & Rummel, 2020 ). Mind wandering refers to a phenomenon in which our attention shifts away from the task at hand toward task - unrelated thoughts (for reviews, see Smallwood et al., 2018 ; Smallwood & Schooler, 2006 , 2015 ). It has been estimated that up to 50% of our waking time is spent mind wandering (Kane et al., 2007 ; Killingsworth & Gilbert, 2010 ). Despite its prevalence, most people view mind wandering from a negative perspective, in which our performance will drop if our mind wanders (for reviews, see Mooneyham & Schooler, 2013 ; Stan & Christoff, 2018 ). Indeed, a number of studies have found a negative association between mind wandering and primary task performance, including poorer performance in daily functioning (McVay et al., 2009 ) and driving (Baldwin et al., 2017 ; Yanko & Spalek, 2014 ). However, studies have also shown a positive relationship between mind wandering and both mood and cognition (e.g., Gable et al., 2019 ; Mazzoni, 2019 ; Welz et al., 2018 ). In order to understand how to minimize the costs of mind wandering and maximize its benefits, it is therefore important to determine what factors regulate its occurrence.
Despite mind wandering inherently involving a shift in mental set, no existing study to our knowledge has explicitly examined the role of cognitive flexibility in mind wandering. In the present article, we consider the potential of cognitive flexibility to help explain the nature of mind wandering and its tendencies. In an effort to advance the field, here we first briefly review and discuss the most prominent theories of mind wandering—the executive failure hypothesis (McVay & Kane, 2009 , 2010 , 2012b ), the decoupling hypothesis (Smallwood & Schooler, 2006 ), the process - occurrence framework (Smallwood, 2013 ), and the resource - control account of sustained attention (Thomson et al., 2015 ). Then, we put forward a new perspective centered around cognitive flexibility that was prompted by findings from several mind - wandering studies in older adults (e.g., Gyurkovics et al., 2018 ; Jordão et al., 2019 ; Niedzwienska & Kvavilashvili, 2018 ) and mind - wandering studies involving task-switching paradigms in young adults (e.g., Arnau et al., 2020 ; Kam & Handy, 2014 ; Thomson et al., 2014 ). According to this new switching perspective, the reason why some populations (e.g., healthy older adults) experience distinct patterns of mind wandering stems from differences in cognitive flexibility, as instances of mind wandering are in fact instances of mental set shifting (see Murray & Krasich, 2020 , for a similar argument). After presenting the evidence supporting this new perspective, we put forward recommendations for future research aimed at further understanding the importance of cognitive flexibility in mind wandering.
Existing theories of mind wandering
Executive failure hypothesis.
According to the executive failure hypothesis (McVay & Kane, 2009 , 2010 , 2012b ), the occurrence of mind wandering represents a failure in the control of executive resources to keep attention on the current task, as the suppression of mind wandering requires executive control. One key form of executive control is working memory. This hypothesis posits that individuals with lower working memory capacity (i.e., those who are less able to hold information in an active, quickly retrievable state; Engle, 2002 ) are less capable of maintaining task focus over extended periods of time and keeping mind wandering at bay, and consequently experience more mind wandering. In support of this hypothesis, studies have found that individuals with lower working memory capacity have higher self - reported mind - wandering rates than individuals with higher working memory capacity (McVay & Kane, 2009 , 2012a , 2012b ; Robison & Unsworth, 2018 ; Unsworth & Robison, 2020 ), and working memory capacity can reliably predict how often one’s mind wanders (Kane et al., 2001 ; Robison & Unsworth, 2018 ). A meta - analysis that examined the association between mind wandering, executive resources (e.g., working memory capacity), and task performance also provided support for this hypothesis, by showing that individuals with lower working memory capacity tend to engage in more mind wandering than individuals with higher working memory capacity (Randall et al., 2014 ).
Additional evidence in support of the executive failure hypothesis comes from research on mind wandering involving individuals with attention - deficit/hyperactivity disorder (ADHD; for a review, see Bozhilova et al., 2018 )—a neurodevelopmental disorder characterized by inattentiveness, hyperactivity, and/or impulsivity (American Psychiatric Association, 2013 ). Using a probe - caught method, in which participants are intermittently interrupted during a vigilance task and probed to report where their attention is focused, Shaw & Giambra, ( 1993 ) showed that participants with a childhood history of ADHD diagnosis reported experiencing more task - unrelated thoughts during task performance than participants with no history of ADHD. Another study, which distinguished between deliberate mind wandering and spontaneous mind wandering, found that the occurrence of spontaneous, but not deliberate, mind wandering is positively associated with ADHD symptom severity (Seli et al., 2015 ). A similar result was obtained by Franklin et al., ( 2017 ), who found that a composite index of ADHD symptoms was positively correlated with both the frequency of mind wandering and a lack of awareness of mind wandering. More recently, Mowlem et al., ( 2019 ) demonstrated that elevated frequencies of mind wandering in adults with ADHD were positively correlated with self - reported measures of functional impairment across major life domains (e.g., school), and that the contribution of mind wandering to their impairment was independent of the core ADHD symptoms (inattention, hyperactivity, and impulsivity). Given that most individuals with ADHD have deficits in a variety of cognitive domains (e.g., Coutinho et al., 2018 ; Kasper et al., 2012 ; Ramos et al., 2020 ), these studies suggest that the excessive mind wandering they experience could be attributable to, at least in part, a failure of executive control (McVay & Kane, 2010 ).
Decoupling hypothesis
The decoupling hypothesis (Smallwood & Schooler, 2006 ) suggests that decreased performance during mind wandering occurs primarily because our attention has become decoupled from the task at hand and is instead coupled to task - unrelated thoughts. This decoupling process is important as it prevents information processing of extraneous stimuli from interfering with our current mental focus (Smallwood et al., 2011 ) in order to ensure continuity of the train of thought (Smallwood, 2013 ). In other words, the decoupling hypothesis proposes that mind wandering is a process that relies on some of the same cognitive mechanisms involved in maintaining focused attention on the task at hand and thus directly competes with primary task performance for executive resources (Smallwood & Schooler, 2006 ).
Several event - related potential (ERP) studies have provided strong evidence for this hypothesis. For example, using the sustained attention to response task (SART; Robertson et al., 1997 ), Smallwood et al., ( 2008 ) showed that participants had a reduced P300 amplitude during self - reported mind wandering relative to on - task episodes. The P300 is a positive potential that peaks around 300 ms after stimulus presentation and is believed to reflect the extent to which the stimulus representation is updated in working memory (Donchin, 1981 ; Donchin & Coles, 1988 ) and/or the amount of executive resources allocated toward the stimulus (Kramer & Strayer, 1988 ; Wickens et al., 1983 ), with higher amplitude indicating more revision of the representations and/or more executive resources directed to processing the stimulus (for reviews, see Polich, 2007 ; Verleger, 2020 ). Because the P300 can provide an index of executive resources (e.g., Kramer & Strayer, 1988 ; Wickens et al., 1983 ), the decreased P300 amplitude during mind wandering indicates that our executive resources have been withdrawn, at least partly, from the primary task and are presumably directed toward task - unrelated thoughts (Smallwood, 2010 ; Smallwood & Schooler, 2006 ). Similar results were obtained in subsequent studies (Baldwin et al., 2017 ; Barron et al., 2011 ; Kam et al., 2014 ; Maillet et al., 2020 ).
Process-occurrence framework
The process - occurrence framework, which was proposed by Smallwood, ( 2013 ), emphasizes the necessity of distinguishing between the onset of mind wandering and the continuation of the mind-wandering episode, linking McVay and Kane’s view (i.e., executive control failure) to the onset and Smallwood and Schooler’s view (i.e., mind wandering requires executive resources) to the continuation of the episode. According to this framework, under tasks requiring sustained attention, executive control can keep mind wandering at bay by ensuring the continuity of the train of task - related thought. However, when mind wandering occurs (e.g., due to executive control failure; McVay & Kane, 2009 , 2010 , 2012b ), executive control shifts away (i.e., decouples) from the task at hand to enable the continuation of the mind-wandering episode (which consumes the same executive resources as the task at hand; Smallwood & Schooler, 2006 ), leaving insufficient executive resources for the primary task, thereby resulting in impaired task performance (Smallwood et al., 2012 ).
As proposed by Smallwood, ( 2013 ), there are at least two reasons why one would mind wander more. First, because the individual has difficulties in ensuring the continuity of their train of thought (see also McVay & Kane, 2010 ). This account could explain why individuals with ADHD tend to experience more mind wandering episodes (Bozhilova et al., 2018 ; Franklin et al., 2017 ; Mowlem et al., 2019 ; Seli et al., 2015 ; Shaw & Giambra, 1993 ). Second, because the individual considers their currently relevant personal concerns or unresolved goals (e.g., submit the assignment before the end of the day) as having higher priority than the demands of the task at hand, and thus shifts their attention toward these concerns (see also Klinger, 1975 , 1999 ). This account could explain why older adults report less mind wandering than young adults (e.g., Jordão et al., 2019 ; Maillet & Schacter, 2016 ), as they tend to report having fewer concerns (Parks et al., 1989 ). In the former case, according to this framework (Smallwood, 2013 ), the individual should experience more frequent mind-wandering episodes, whereas in the latter case, the individual should experience longer episodes of mind wandering.
Although it is difficult to identify different states and processes involved in mind wandering, primarily because people normally do not realize when they first start mind wandering but only notice some time later (Smallwood, 2013 ; Zukosky & Wang, 2021 ), there has been one study to date that made an attempt at this (Voss et al., 2018 ). In this study, the researchers characterized the mind-wandering process by combining the self-caught and the probe-caught methods to estimate the duration of focus (defined as the time period from when the person first started focusing on the task at hand to the moment that mind wandering began), which was taken as a measure of one’s ability to maintain task focus and resist the occurrence of mind wandering, and the duration of mind wandering (defined as the time period from when the mind-wandering episode began to the moment that the individual caught themselves and reported it by pressing a button), which was taken as a measure of processes that keep one in the mind-wandering state. The researchers then investigated the association of these measures with working memory capacity. The results showed a strong positive correlation between the duration of focus and working memory capacity, which is consistent with previous findings that individuals with higher working memory capacity could maintain task focus over longer periods of time (Kane et al., 2001 ; McVay & Kane, 2009 , 2012a , 2012b ; Randall et al., 2014 ; Robison & Unsworth, 2018 ; Unsworth & Robison, 2020 ). However, no relationship was observed between the duration of mind wandering and working memory capacity, indicating that one’s tendency to engage in and detect the mind-wandering state was not affected by working memory capacity. The Voss et al., ( 2018 ) study, therefore, provides initial evidence to support the process - occurrence framework (Smallwood, 2013 ) that there are at least two distinct states and processes involved in mind wandering.
Resource-control account of sustained attention
According to the resource - control account of sustained attention (Thomson et al., 2015 ), mind-wandering state is the default mental state and thus there is a continuous bias for executive resources to be directed toward mind wandering (see also Baird et al., 2011 ; Smallwood, 2013 ; Smallwood et al., 2009 ). This theory posits that the occurrence of mind wandering should be associated with decreases in motivation and/or effort to keep attention on the task at hand over time. In other words, as time-on-task increases, executive resources are less likely to be allocated to the task at hand and are more likely to be allocated to mind wandering, leaving insufficient executive resources for the primary task and thereby resulting in impaired task performance. In support of this theory, studies have found a negative association between time on task and primary task performance, and a positive association between time on task and rates of self - reported mind wandering (Brosowsky et al., 2020 ; Krimsky et al., 2017 ; McVay & Kane, 2012a ; Thomson et al., 2014 ).
Interim summary
Taken together, these four theories suggest that mind wandering is a state of decoupled information processing (Smallwood and Schooler’s view) that involves at least two component processes (Smallwood’s view): the initiation of mind wandering, which can be attributed to a failure of executive control (McVay and Kane’s view), and the continuation of the mind-wandering episode, which is a resource-dependent process (Smallwood and Schooler’s and Thomson et al.’s view). Although these four theories significantly advance our understanding of mind wandering, they are not without weaknesses or alternative interpretations. In the next section, we discuss these and put forward a new perspective of mind wandering focused on cognitive flexibility, which offers novel insight that aids towards our general understanding of mind wandering.
Insight from a switching perspective
Cognitive flexibility, which can also be referred to as mental set shifting or switching, is one of the three core executive control functions (along with inhibitory control and working memory) that enables us to adjust our thoughts and actions in response to changed priorities or demands (Buttelmann & Karbach, 2017 ; Diamond, 2013 ; Miyake et al., 2000 ). To adapt to changing priorities, for example, we need to inhibit previously relevant thoughts and actions and activate newly relevant thoughts and actions in working memory. In this way, mental set shifting requires involvement of both inhibitory control and working memory (Diamond, 2013 ). With regard to mind wandering, we propose that it requires cognitive flexibility, as the occurrence of mind wandering entails inhibition of one’s primary task mental set (which enables decoupling to occur) and activation of task - unrelated thoughts in working memory (see Fig. 1 ).
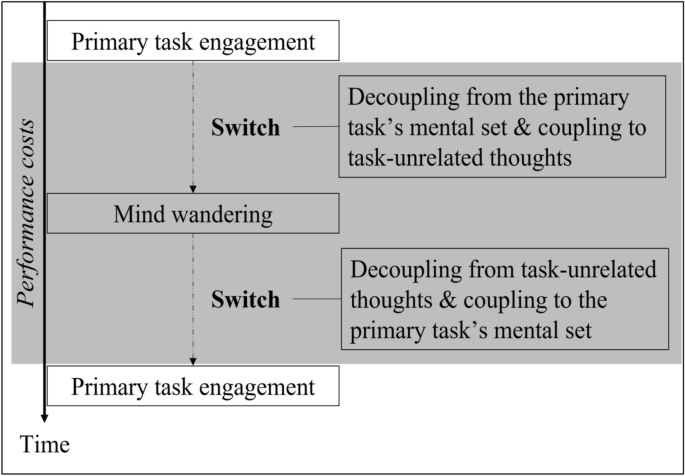
Conceptual framework for viewing mind wandering from a switching perspective. Maintenance of both primary task and mind - wandering mental sets occur in working memory. Each change of mental set requires inhibition of the previously relevant mental set. Grey area represents the time in which primary task performance costs arise
Considering this switching view alongside existing frameworks and models such as the metacontrol state model (Hommel, 2015 ), which describes the balance between flexibility and persistence in cognitive processing (Zhang et al., 2020 ), may provide a way to understand variability in mind-wandering frequency. For instance, given that ADHD has long been found to be associated with dysregulated dopamine neurotransmission (Cook et al., 1995 ), and dopamine-related interindividual differences have been hypothesized to be associated with interindividual variability in metacontrol defaults (i.e., the control of the current cognitive-control settings; Hommel & Colzato, 2017 ), it seems possible that the increased mind wandering experienced by individuals with ADHD might be associated with a default metacontrol setting biased towards flexibility (i.e., weak goal shielding and weak mutual inhibition of task-related and task-unrelated mental sets) that may be related to imbalances of neurotransmitters. Moreover, given that older adults tend to report higher levels of positive affect (e.g., Frank et al., 2015 ) and motivation (e.g., Nicosia & Balota, 2021 ; Ryan & Campbell, 2021 ; Seli et al., 2021 ) than young adults during task performance, and situational factors such as these have been hypothesized to induce a metacontrol setting biased towards persistence (Hommel & Colzato, 2017 ), it seems possible that older adults’ less frequent reports of mind wandering (e.g., Arnicane et al., 2021 ; Jordão et al., 2019 ; Maillet & Schacter, 2016 ) might be associated with a stronger bias towards persistence (i.e., stronger goal shielding and stronger mutual inhibition of task-related and task-unrelated mental sets).
It may also be possible to account for the effect of mind wandering on creativity (e.g., Gable et al., 2019 ; Murray et al., 2021 ; Steindorf et al., 2021 ; Yamaoka & Yukawa, 2020 ) by integrating the switching perspective of mind wandering with both the metacontrol state model (Hommel, 2015 ) and the dynamic framework of thought (Christoff et al., 2016 )—a model that provides insight into how thoughts that focus on personally or affectively salient information (i.e., automatic constraints) and thoughts that focus on goal-related information (i.e., deliberate constraints) dynamically influence the nature of thought over time. For example, given that both of these schemas emphasize the importance of flexibility or shifting between mental states in idea generation (for reviews, see Girn et al., 2020 ; Zhang et al., 2020 ), it seems possible that the relationship between mind wandering and creative thinking might be mediated by cognitive flexibility. This speculation may be worthy of future research.
In short, we believe that viewing mind wandering from a switching perspective may help explain, at least in part, why some populations experience more mind-wandering episodes while others experience fewer episodes, why participants who indicate higher levels of motivation are less likely to engage in mind wandering during task performance, and why mind wandering is sometimes linked to enhanced creativity.
Limitations and an alternative viewpoint for the executive failure hypothesis
Although the executive failure hypothesis (McVay & Kane, 2009 , 2010 , 2012b ), which posits that working memory plays a critical role in keeping mind wandering at bay, could explain the higher levels of mind wandering in healthy young adults with lower working memory capacity and individuals with ADHD, mind-wandering research involving other cohorts with lower working memory capacity has yielded results that challenge this account. For instance, a meta - analysis of 21 studies investigating aging effects in mind wandering revealed that older adults tend to report fewer instances of mind wandering when engaged in cognitive tasks (Jordão et al., 2019 ). This is puzzling given that according to the executive failure hypothesis, one would expect the rates of mind wandering to increase—not decrease—in older adults (for a review, see Maillet & Schacter, 2016 ), as executive control functions generally decline with advancing age (e.g., Craik & Salthouse, 2011 ; Foster et al., 2007 ; Machado, 2021 ). Furthermore, using the SART, Gyurkovics et al. ( 2018 ) found that individuals with early-stage Alzheimer’s disease reported experiencing fewer task - unrelated thoughts and more task - related thoughts than healthy age - matched controls, again indicating reduced incidence of mind wandering despite individuals with Alzheimer's disease showing declines in executive functioning (Guarino et al., 2018 ). Similar results have been reported in studies involving individuals with Parkinson’s disease (Walpola et al., 2020 ), who are also known to suffer from executive dysfunction (Flannery et al., 2018 ; McKinlay et al., 2010 ; Ramos & Machado, 2021 ), and individuals with amnestic mild cognitive impairment (Niedzwienska & Kvavilashvili, 2018 ).
A counterargument to the claim that lower levels of mind wandering in these older populations stand against the executive failure hypothesis stems from the fact that mind - wandering studies mostly rely on self - report measures. In relation to this, some researchers have attributed the finding of reduced mind wandering in healthy and cognitively impaired older adults to a lack of validity of their mind - wandering reports (Gyurkovics et al., 2018 ; Jackson & Balota, 2012 ; Zavagnin et al., 2014 ). However, several studies have demonstrated that these populations’ self - reported mind-wandering data are as valid as those by controls, by demonstrating that during self - reported off - task episodes, the two groups exhibited similar levels of disrupted task performance (e.g., Arnicane et al., 2021 ; McVay et al., 2013 ; Niedzwienska & Kvavilashvili, 2018 ). Moreover, eye movement (Frank et al., 2015 ) and brain activation (Maillet & Rajah, 2016 ; Walpola et al., 2020 ) patterns reliably predicted self - reported mind - wandering episodes in older adults and individuals with Parkinson’s disease, indicating that they were able to report their mind - wandering episodes accurately. These results, therefore, suggest that the explanation that decreased mind wandering relates to lack of validity of mind-wandering measures in these older populations does not hold up.
To shed light on decreased mind wandering in older cohorts, here we offer an alternative account of mind wandering focused on cognitive flexibility (for a summary of other alternative explanations for age-related declines in mind wandering, see Seli, Maillet, et al., 2017 ; Seli, Ralph, et al., 2017 ). According to this account, the reduced mind - wandering frequency seen in healthy older adults and those with Alzheimer’s disease, Parkinson’s disease, and amnestic mild cognitive impairment could be attributable, at least partly, to declines in cognitive flexibility (e.g., a reduced ability to switch between task - related and task - unrelated thoughts; see Fig. 2 ). This line of reasoning fits well with research showing that these older populations generally have a reduced capability to activate (e.g., to initiate a switch of mental set) and maintain cognitive representations (e.g., to maintain the new mental set; Craik & Salthouse, 2011 ; Lindenberger & Mayr, 2014 ; Traykov et al., 2007 ), and exhibit longer response times and/or higher error rates on switch trials relative to repetition trials (e.g., Brett & Machado, 2017 ; Rey-Mermet & Gade, 2018 ; Wasylyshyn et al., 2011 ).
Summary of results gathered from several mind-wandering, working memory, and task-switching studies . AD Alzheimer’s disease, PD Parkinson’s disease, aMCI amnestic mild cognitive impairment, WMC working memory capacity. Red circle represents poorer performance and green circle represents better performance, relative to the comparison group. White thought bubble cloud represents mind-wandering frequency, with fewer clouds representing less frequent occurrences of mind wandering, relative to the comparison group
Furthermore, given that previous research in healthy young adults has revealed a negative association between working memory capacity and mind - wandering frequency (McVay & Kane, 2009 , 2012b ; Robison & Unsworth, 2018 ; Unsworth & Robison, 2020 ), and a negative correlation between working memory capacity and task - switching performance (Miyake et al., 2000 ; Oberauer et al., 2003 ; Shipstead et al., 2015 ; for more details, see Draheim et al., 2016 ), it seems plausible that healthy young adults with lower working memory capacity might be more capable of adjusting their executive resources to different mental sets (including task-unrelated mental sets) due to their superior switching abilities (see the “ Future directions ” for further elaboration of this conjecture; see Fig. 2 ). In short, although the executive failure hypothesis (McVay & Kane, 2009 , 2010 , 2012b ) holds up quite well for younger populations, the switching account of mind wandering put forward here could potentially also explain the patterns of results in these populations. Moreover, in contrast to the executive failure hypothesis which cannot account for the lower levels of mind wandering observed in older populations (as it predicts that these populations should exhibit elevated levels of mind wandering due to having lower working memory capacity), our switching account of mind wandering fits well with the existing data, suggesting that cognitive flexibility may play a more important role than working memory does in mediating and/or regulating the occurrence of mind wandering.
An alternative interpretation for the decoupling hypothesis
Although studies using ERPs have provided evidence in favor of the decoupling hypothesis (Smallwood & Schooler, 2006 ) by demonstrating that mind wandering reduces the cortical processing of the task at hand (as reflected by the reduced P300 amplitude during mind wandering; Baldwin et al., 2017 ; Barron et al., 2011 ; Kam et al., 2014 ; Maillet et al., 2020 ; Smallwood et al., 2008 ), these findings could also be interpreted from a switching perspective. Previous research on task switching has consistently revealed a reduced P300 amplitude on switch trials relative to repetition trials (e.g., Barcelo et al., 2002 ; Hsieh & Cheng, 2006 ; Kieffaber & Hetrick, 2005 ; Mueller et al., 2007 ; Poljac & Yeung, 2014 ; Vandamme et al., 2010 ; Wylie et al., 2003 ). According to Jost et al. ( 2008 ), the reduced P300 amplitude in switch trials is thought to indicate that context updating (i.e., the comparison of the attributes of an incoming stimulus with an internal representation and the subsequent updating of the internal representation; Donchin, 1981 ; Donchin & Coles, 1988 ) is less easily achieved. Another explanation comes from Wylie et al., ( 2003 ), who suggested that the reduced P300 amplitude reflects a competition between task sets or rules, with the idea being that on switch trials the competition between task - specific response sets or rules is greater. This results in both a reduction in P300 amplitude and an increase in response time and/or error rate because the activation of the currently relevant task representation is less enhanced. Using the findings from these task - switching studies as a foundation, we posit that the attenuated P300 amplitude during mind wandering could reflect two possible processes, including ( a ) less efficient context updating in working memory (Donchin, 1981 ; Donchin & Coles, 1988 ), and/or ( b ) competition between primary-task representations and task - unrelated thoughts.
Building on this alternative perspective, we suggest that the decoupling process is a component of the switching process. As such, mind wandering could be considered as a subset of task switching that typically would run serially with task performance (i.e., serial multitasking; e.g., Huijser et al., 2018 ; for more details, see Taatgen et al., 2021 ), although a recent study has shown that mind wandering can also run parallelly with particular kinds of tasks (i.e., parallel multitasking; e.g., Brosowsky et al., 2021 ). Furthermore, in the context of serial processing of multiple mental sets, switch costs should be observed regardless of whether the shift is from external to internal (e.g., shifting from a SART to task-irrelevant personal concerns), internal to external (e.g., shifting from task-irrelevant personal concerns back to the SART), or internal to internal (e.g., shifting from mental arithmetic to task-irrelevant personal concerns). According to our view, the detrimental effect of mind wandering on primary task performance reflects the costs of switching between mental sets (i.e., decoupling from the primary task’s mental set and coupling to task - unrelated thoughts; see Fig. 1 ), in addition to the costs of not paying attention to the primary task while decoupled. This proposal is in line with research on task switching that demonstrated that switch costs could still be observed when the tasks were relatively simple, when the task sequence was predictable, and when there was a cue signaling the upcoming switch (Koch, 2003 ).
A possible way to test the process-occurrence framework
Within the process-occurrence framework, Smallwood aptly noted the following:
…the processes that ensure the continuity of the experience of an internal train of thought are similar to those that can be engaged in standard task-relevant paradigms, and as a result, these processes are becoming reasonably well understood. By contrast, our understanding of why mind wandering occurs is less well specified, in part because we are unable to identify the moment of ignition for the state. (Smallwood, 2013 , p. 532).
Indeed, as mentioned earlier, one major challenge in investigating the length of mind-wandering episodes is how to determine the “when” of mind wandering (Franklin et al., 2013 ). Although Voss et al., ( 2018 ) have provided evidence in favor of the process-occurrence framework by identifying different states and processes of mind wandering (see the “ Process-occurrence framework ” for more details), one key limitation of this study, as pointed out by the researchers themselves, was that their assessment methods hinged on the assumption that the only way for an individual to redirect their attention from mind wandering back to the task at hand is through a mechanism reliant upon self-awareness (i.e., the meta-awareness system; Schooler et al., 2011 ). If one can return to a task-focused state without relying on such a mechanism (e.g., decoupling from task-unrelated thoughts and coupling to the primary task’s mental set without conscious awareness), and can have multiple switches between task-focused and mind-wandering states during a single self-caught episode, then the estimated duration of focus and mind-wandering episodes (defined earlier in the “ Process-occurrence framework ”) could in fact reflect multiple focus-mind-wandering episodes, rather than the duration of each individual task-focused/mind-wandering state (Voss et al., 2018 ).
To shed light on how to measure the component processes of mind wandering while acknowledging that mind wandering often consumes executive resources that are needed to perform the task (i.e., parallel processing of both high cognitive demand tasks and mind wandering is difficult to achieve; Smallwood & Schooler, 2006 ; Thomson et al., 2015 ), here we put forward a new approach that links the onset of mind wandering to the onset of a new mental set, and the continuation of the mind-wandering episode to the continuation of the new mental set. Previous task-switching studies have identified a number of distinct cognitive processes underlying an attentional set switch (e.g., Meiran et al., 2000 ; Rushworth et al., 2002 , 2005 ). For instance, Rushworth et al., ( 2002 ) found that mental set shifting consisted of at least three component cognitive processes, including: ( a ) initiation of a new mental set prior to selective focusing of attention, which was indexed by an early period of ERP modulation associated with dipole source estimates in the prefrontal cortex; ( b ) reconfiguration of the new mental set, which was indexed by a later period of ERP modulation associated with dipole source estimates at the ventromedial occipitotemporal junction; and ( c ) maintenance of the new mental set and possible interference from the previous mental set, which was indexed by the N200—a negative potential over the central posterior scalp that peaks around 200 ms after stimulus presentation and is believed to be associated with response suppression (Eimer, 1993 ; Kok, 1986 ; Patel & Azzam, 2005 ). Building on these findings, we posit that at the start of the mind-wandering episode, there should be activation in the prefrontal cortex (for a review, see Zamani et al., 2022 )—a region that has been found to play a central role in cognitive flexibility (Dove et al., 2000 ; Miller & Buschman, 2013 ; Miller & Cohen, 2001 ; Sakai & Passingham, 2003 ; Sohn et al., 2000 ) and mind wandering (Bertossi & Ciaramelli, 2016 ; Burgess et al., 2007 ; Christoff et al., 2009 ; Fox et al., 2015 ; Stawarczyk & D’Argembeau, 2015 ).
Using these findings as a foundation, it seems possible that the “when” of mind wandering (i.e., the onset of mental set shifting) can be estimated, at least approximately, from activity in the prefrontal cortex measured prior to periods of self-reported mind wandering. This conjecture seems to fit with findings that non-invasive transcranial direct current stimulation of the prefrontal cortex can increase the propensity to mind wander (e.g., Axelrod et al., 2015 , 2018 ; Filmer et al., 2019 ). To clarify, we postulate that positive-polarity stimulation “encourages” the recipient to initiate a switch of mental set, which according to the resource-control account of sustained attention (Thomson et al., 2015 ) is most likely to involve a switch to a task-unrelated mental set as mind wandering is thought to be the default mental state for most individuals.
Limitations and a possible extension for the resource-control account of sustained attention
Although the resource-control account of sustained attention (Thomson et al., 2015 ), which suggests that the occurrence of mind wandering is associated with decreases in motivation and/or effort to keep attention on the task at hand over time, could explain why older adults tend to report fewer instances of mind wandering than young adults during cognitive task performance—either because they are more motivated to perform the primary task (Frank et al., 2015 ; Jackson & Balota, 2012 ; Seli et al., 2021 ; Seli, Maillet, et al., 2017 ; Seli, Ralph, et al., 2017 ) or because they have spent a larger proportion of their executive resources on the primary task (Craik & Byrd, 1982 ) and thus have fewer resources left over to exhibit mind wandering (Giambra, 1989 ; Krawietz et al., 2012 ; Maillet & Rajah, 2013 )—this theory is not without its limitations. In particular, if executive control, which wanes over time on task, is required to prevent task-unrelated thoughts (i.e., the default mental state) from consuming executive resources needed for the task at hand, then given that healthy and cognitively impaired older adults generally have poorer executive control (e.g., Flannery et al., 2018 ; Guarino et al., 2018 ; McKinlay et al., 2010 ; Ramos & Machado, 2021 ), one might reasonably expect that as time-on-task increases, these older populations would report higher incidences of mind wandering and show more pronounced performance decrements. However, this prediction was not supported by Arnicane et al., ( 2021 ), who found that in comparison to the first block (i.e., the first 15 min) of a visual working memory task, in the sixth block healthy older adults reported similar levels of attentional lapses and demonstrated improved performance. These results, therefore, are inconsistent with the predictions of the resource-control account of sustained attention, as they showed that extended task duration in fact has positive effects on healthy older adults’ working memory performance.
Here, we posit that the occurrence of mind wandering should be also associated with fluctuations in activity in brain regions associated with executive control (the frontal-parietal and dorsal attention networks; Corbetta & Shulman, 2002 ; Corbetta et al., 2008 ; Posner & Dehaene, 1994 ; Vincent et al., 2008 ) and mind wandering (the default mode network; Raichle et al., 2001 ), and that these fluctuations should be inversely related (see also Esterman & Rothlein, 2019 ). According to this account, because normal aging is associated with significant decreases in the strength of functional connectivity density (i.e., the statistical relationship between brain regions; Tomasi & Volkow, 2010 ) in the dorsal attention and default mode networks (Tomasi & Volkow, 2012 ), the lower frequencies of mind wandering reported in healthy older adults could be attributable to less efficient switching and/or cooperation between these two networks to produce a train of thought during mind wandering (Smallwood et al., 2012 ). This proposal goes beyond the resource-control account of sustained attention, which cannot account for the findings that longer task duration does not lead to a higher incidence of mind wandering or more pronounced performance decrements in healthy older adults (Arnicane et al., 2021 ). In support of this proposal, studies have shown that mind wandering is associated with increased default mode network and decreased dorsal attention network activation (Christoff et al., 2009 ; Fortenbaugh et al., 2018 ; Kucyi et al., 2013 ; Mason et al., 2007 ; Robertson et al., 1997 ; Smallwood et al., 2013 ), indicating that there might be a push–pull relationship between these two networks that impacts the occurrence of mind wandering (cf. Esterman & Rothlein, 2019 ).
We have shown that the switching perspective is a useful addition to the four prominent theories of mind wandering. While acknowledging that other factors may be at play, this newly formulated view not only provides a plausible explanation as to why healthy and cognitively impaired older adults experience a reduction in mind wandering, but it also provides new insights for determining the initiation of mind-wandering episodes. In the next section, we present evidence to support our view.
Review of evidence supporting the switching perspective
The strongest evidence to date in support of this new perspective comes from research using the voluntary task - switching paradigm (Arrington & Logan, 2004 ), for which participants are free to switch tasks or continue with the same task at their preference. Somewhat paradoxically, research has consistently demonstrated that most of the participants decided to switch tasks despite negative consequences (i.e., switch costs; e.g., Irons & Leber, 2016 ; Kessler et al., 2009 ; Mittelstädt et al., 2019 ), although comparatively healthy older adults tended to initiate voluntary task switching less frequently than healthy young adults (Ardiale & Lemaire, 2012 ; Butler & Weywadt, 2013 ; Lockenhoff et al., 2020 ; Terry & Sliwinski, 2012 ). In light of cognitive aging, this finding may not seem surprising given that repeating the currently active task set requires fewer executive resources than switching to a different task set (Wirth et al., 2018 ) and switching between task sets or rules increases cognitive load (Arrington & Logan, 2004 ; Kool et al., 2010 ). In like manner, we argue that it should not be surprising either that healthy older adults and older adults with Alzheimer’s disease, Parkinson’s disease, and amnestic mild cognitive impairment experience less frequent mind wandering, as a reduced switching ability could contribute, at least in part, to getting “stuck” in a task - focus mode (cf. Walpola et al., 2020 ).
Additional evidence for the switching perspective of mind wandering comes from the intriguing finding that mind wandering does not always impair young adults’ performance in switching tasks, in contrast to tasks that require sustained attention. Using a probe - caught method, Kam & Handy, ( 2014 ) found no significant disruptive effects of mind wandering on task-switching performance. A further study, which investigated the association between mind wandering and task-switching performance over time, observed similar response times and accuracy rates for the trials leading up to “on-task” and “off-task” reports (Thomson et al., 2014 ), which again suggests that switching performance was unaffected by mind wandering. Similar results were obtained by Arnau et al., ( 2020 ), who investigated electrophysiological correlates of mind wandering during a switching task and did not observe slower response times during periods of self-reported mind wandering relative to on-task episodes.
The lack of performance costs, particularly response time costs, for switching tasks is puzzling because mind wandering has consistently been found to disrupt behavioral performance on tasks that tap the other two core executive function measures—inhibition (e.g., Kam & Handy, 2014 ; Smallwood et al., 2008 ; Stawarczyk et al., 2011 ) and working memory (e.g., Kam & Handy, 2014 ; Krimsky et al., 2017 ; Unsworth & Robison, 2016 ). Given this, one might expect that mind wandering should also significantly affect one’s task-switching performance. Although Kam & Handy, ( 2014 ) speculated that the null effect of mind wandering on switching-task performance might reflect cognitive flexibility being a less representative executive functioning skill (as it showed the weakest correlations with other executive function measures; for more details, see Miyake et al., 2000 ), these researchers also noted that switching from the task at hand to task-unrelated thoughts may be a form of switching. In the same manner, we posit that because switching either between task-related mental sets or between task-related and task-unrelated mental sets requires cognitive flexibility, when one mind wanders during performance of a switching task, they continue to engage in a “task-switching mind frame” (i.e., instead of switching between task-related mental sets, the individual switches between task-related and task-unrelated mental sets), and thereby can maintain task-switching performance. This conjecture appears to fit well with previous studies indicating that frequent task/response switches can shift the flexibility-persistence balance (Hommel, 2015 ) towards higher flexibility (e.g., Fröber & Dreisbach, 2017 ; Fröber et al., 2018 ; Zhuo et al., 2021 ; for a review, see Dreisbach & Fröber, 2019 ).
Future directions
Acknowledging that instances of mind wandering are instances of mental set shifting (see Murray & Krasich, 2020 , for a similar argument) opens up new avenues for future scientific investigations. First, to directly investigate this new perspective, future research could examine the association between cognitive flexibility and the tendency to mind wander, as despite a number of researchers using a task - switching paradigm as the primary task in their investigation of mind wandering (e.g., Arnau et al., 2020 ; Kam & Handy, 2014 ; Thomson et al., 2014 ), to our knowledge none have explicitly investigated the role of cognitive flexibility in mind wandering. Second, as there are many different types of switching (e.g., rule switching, task set switching, and response set switching), which have been found to activate different brain areas (Ravizza & Carter, 2008 ), it may be important to investigate how these switching abilities are related and which type is most closely associated with mind wandering. Determining this may help advance the current understanding of the higher incidence of mind wandering in ADHD, and may ultimately shed light on the inconsistent results regarding whether ADHD is associated with deficits in cognitive flexibility (e.g., Halleland et al., 2012 ; Irwin et al., 2019 ; Rohlf et al., 2012 ; Willcutt et al., 2005 ), which in turn may shed further light on the viability of the switching perspective forwarded here. Third, as an increasing number of studies have revealed distinct effects of intentional and unintentional mind wandering on task performance (e.g., Martínez-Pérez et al., 2021 ; Moran et al., 2021 ; Seli et al., 2016a , 2016b ; for a review, see Seli, et al., 2016a , 2016b ), future research could investigate whether intentional and unintentional mind wandering constitute distinct forms of task-set activation (e.g., counscious or uncounscious activation of task-unrelated mental sets; Arango-Muñoz & Bermúdez, 2021 ; Lau & Passingham, 2007 ; Reuss et al., 2011 ; Weibel et al., 2013 ) that involve distinct neural mechanisms and might differ with respect to their relationships with cognitive flexibility.
Fourth, considering that in a real-world setting we constantly multitask (e.g., writing an email while listening to music and eating a meal), and studies have found a positive association between self-reported frequency of concurrent use of multiple digital media streams and mind-wandering tendency (e.g., Kane et al., 2017 ; Ralph et al., 2014 ; Wiradhany & Koerts, 2021 ), future research could investigate the association between mind wandering, multitasking, and cognitive flexibility. Fifth, to understand past findings (e.g., McVay & Kane, 2009 ; Robison & Unsworth, 2018 ; Unsworth & Robison, 2020 ) in light of this switching perspective, future studies could investigate whether healthy young adults with higher self-reported mind-wandering tendencies have lower working memory capacity but superior switching abilities. In consideration of previous findings (e.g., McVay & Kane, 2009 , 2012b ; Miyake et al., 2000 ; Oberauer et al., 2003 ; Robison & Unsworth, 2018 ; Shipstead et al., 2015 ; Unsworth & Robison, 2020 ; as discussed in the “ Limitations and an alternative viewpoint for the executive failure hypothesis ”), the increased mind - wandering frequency seen in healthy young adults with lower working memory capacity could reflect a tendency to initiate more switches between task-related and task-unrelated mental sets in relation to superior switching abilities. Sixth, considering that the executive failure hypothesis (McVay & Kane, 2009 , 2010 , 2012b ) fits with the data in healthy young adults and individuals with ADHD, but does not account for the data in older adult populations discussed in this review, future research should explore the association between cognitive flexibility and the tendency to mind wander in different populations with age in mind, as it could be the case that the executive failure hypothesis applies to young adults whereas the switching account of mind wandering applies to older populations.
Concluding remarks
The findings reviewed in this article provide initial evidence to suggest that there may be an association between cognitive flexibility and mind wandering, and that distinct patterns of mind wandering may signal and be a product of altered cognitive flexibility. Although more research is needed, the switching perspective of mind wandering put forward here may provide a more comprehensive account of mind wandering that fits better with the experimental findings to date, including why healthy older adults and those with Alzheimer’s disease, Parkinson’s disease, and amnestic mild cognitive impairment experience less mind wandering (e.g., Gyurkovics et al., 2018 ; Jordão et al., 2019 ; Maillet & Schacter, 2016 ; Niedzwienska & Kvavilashvili, 2018 ; Walpola et al., 2020 ). This novel line of research may lead to the development of clinical detection tools and therapeutic approaches (e.g., task - switching training) aimed at preserving, or enhancing, the rates of mind wandering in populations with reduced levels of mind wandering (e.g., older adults), as mind wandering does have important functions such as facilitating future planning (e.g., Baird et al., 2011 ; Mazzoni, 2019 ; Seli, Maillet, et al., 2017 ; Seli, Ralph, et al., 2017 ; Stawarczyk et al., 2011 ) and the generation of creative ideas (e.g., Gable et al., 2019 ; Yamaoka & Yukawa, 2020 ) as well as promoting positive mood (e.g., Welz et al., 2018 ). Furthermore, recognizing that previous findings on mind wandering can be viewed from a switching perspective may provide an important contribution to our understanding of the basic psychological processes of mind wandering and its determinants, and may help future research to come up with a definition of mind wandering that will gain consensus in the field.
Data availability
Not applicable.
Code availability
American Psychiatric Association. (2013). Neurodevelopmental Disorders. In: Diagnostic and statistical manual of mental disorders, 5th edn. https://doi.org/10.1176/appi.books.9780890425596.dsm01
Arango-Muñoz, S., & Bermúdez, J. P. (2021). Intentional mind-wandering as intentional omission: The surrealist method. Synthese, 199 (3), 7727–7748. https://doi.org/10.1007/s11229-021-03135-2
Article PubMed PubMed Central Google Scholar
Ardiale, E., & Lemaire, P. (2012). Within-item strategy switching: an age comparative study in adults. Psychology and Aging, 27 (4), 1138–1151. https://doi.org/10.1037/a0027772
Article PubMed Google Scholar
Arnau, S., Löffler, C., Rummel, J., Hagemann, D., Wascher, E., & Schubert, A. L. (2020). Inter-trial alpha power indicates mind wandering. Psychophysiology, 57 (6), e13581. https://doi.org/10.1111/psyp.13581
Arnicane, A., Oberauer, K., & Souza, A. S. (2021). Validity of attention self-reports in younger and older adults. Cognition, 206 , 104482. https://doi.org/10.1016/j.cognition.2020.104482
Arrington, C. M., & Logan, G. D. (2004). The cost of a voluntary task switch. Psychological Science, 15 (9), 610–615. https://doi.org/10.1111/j.0956-7976.2004.00728.x
Axelrod, V., Rees, G., Lavidor, M., & Bar, M. (2015). Increasing propensity to mind-wander with transcranial direct current stimulation. Proceedings of the National Academy of Sciences, 112 (11), 3314–3319. https://doi.org/10.1073/pnas.1421435112
Article Google Scholar
Axelrod, V., Zhu, X., & Qiu, J. (2018). Transcranial stimulation of the frontal lobes increases propensity of mind-wandering without changing meta-awareness. Scientific Reports, 8 (1), 15975. https://doi.org/10.1038/s41598-018-34098-z
Baird, B., Smallwood, J., & Schooler, J. W. (2011). Back to the future: Autobiographical planning and the functionality of mind-wandering. Consciousness and Cognition, 20 (4), 1604–1611. https://doi.org/10.1016/j.concog.2011.08.007
Baldwin, C. L., Roberts, D. M., Barragan, D., Lee, J. D., Lerner, N., & Higgins, J. S. (2017). Detecting and quantifying mind wandering during simulated driving. Frontiers in Human Neuroscience, 11 , 406. https://doi.org/10.3389/fnhum.2017.00406
Barcelo, F., Perianez, J. A., & Knight, R. T. (2002). Think differently: A brain orienting response to task novelty. NeuroReport, 13 (15), 1887–1892. https://doi.org/10.1097/00001756-200210280-00011
Barron, E., Riby, L. M., Greer, J., & Smallwood, J. (2011). Absorbed in thought: The effect of mind wandering on the processing of relevant and irrelevant events. Psychological Science, 22 (5), 596–601. https://doi.org/10.1177/0956797611404083
Bertossi, E., & Ciaramelli, E. (2016). Ventromedial prefrontal damage reduces mind-wandering and biases its temporal focus. Social Cognitive and Affective Neuroscience, 11 (11), 1783–1791. https://doi.org/10.1093/scan/nsw099
Bozhilova, N. S., Michelini, G., Kuntsi, J., & Asherson, P. (2018). Mind wandering perspective on attention-deficit/hyperactivity disorder. Neuroscience and Biobehavioral Reviews, 92 , 464–476. https://doi.org/10.1016/j.neubiorev.2018.07.010
Brett, C. H. R., & Machado, L. (2017). Manual versus saccadic assessment of cognitive inhibition and switching in young and older adults. Psychological Assessment, 29 (11), 1420–1425. https://doi.org/10.1037/pas0000453
Brosowsky, N. P., DeGutis, J., Esterman, M., Smilek, D., & Seli, P. (2020). Mind wandering, motivation, and task performance over time: Evidence that motivation insulates people from the negative effects of mind wandering. Psychology of Consciousness: Theory, Research, and Practice . https://doi.org/10.1037/cns0000263
Brosowsky, N. P., Murray, S., Schooler, J. W., & Seli, P. (2021). Attention need not always apply: mind wandering impedes explicit but not implicit sequence learning. Cognition, 209 , 104530. https://doi.org/10.1016/j.cognition.2020.104530
Burgess, P. W., Dumontheil, I., & Gilbert, S. J. (2007). The gateway hypothesis of rostral prefrontal cortex (area 10) function. Trends in Cognitive Sciences, 11 (7), 290–298. https://doi.org/10.1016/j.tics.2007.05.004
Butler, K. M., & Weywadt, C. (2013). Age differences in voluntary task switching. Psychology and Aging, 28 (4), 1024–1031. https://doi.org/10.1037/a0034937
Buttelmann, F., & Karbach, J. (2017). Development and plasticity of cognitive flexibility in early and middle childhood. Frontiers in Psychology, 8 , 1040. https://doi.org/10.3389/fpsyg.2017.01040
Christoff, K., Gordon, A. M., Smallwood, J., Smith, R., & Schooler, J. W. (2009). Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proceedings of the National Academy of Sciences, 106 (21), 8719–8724. https://doi.org/10.1073/pnas.0900234106
Christoff, K., Irving, Z. C., Fox, K. C. R., Spreng, R. N., & Andrews-Hanna, J. R. (2016). Mind-wandering as spontaneous thought: A dynamic framework. Nature Reviews Neuroscience, 17 (11), 718–731. https://doi.org/10.1038/nrn.2016.113
Cook, E. H., Jr., Stein, M. A., Krasowski, M. D., Cox, N. J., Olkon, D. M., Kieffer, J. E., & Leventhal, B. L. (1995). Association of attention-deficit disorder and the dopamine transporter gene. American Journal of Human Genetics. 56(4), 993–998 https://pubmed.ncbi.nlm.nih.gov/7717410
Corbetta, M., & Shulman, G. L. (2002). Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience, 3 (3), 201–215. https://doi.org/10.1038/nrn755
Corbetta, M., Patel, G., & Shulman, G. L. (2008). The reorienting system of the human brain: From environment to theory of mind. Neuron, 58 (3), 306–324. https://doi.org/10.1016/j.neuron.2008.04.017
Coutinho, T. V., Reis, S. P. S., da Silva, A. G., Miranda, D. M., & Malloy-Diniz, L. F. (2018). Deficits in response inhibition in patients with attention-deficit/hyperactivity disorder: the impaired self-protection system hypothesis. Frontiers in Psychiatry, 8 , 299. https://doi.org/10.3389/fpsyt.2017.00299
Craik, F. I. M., & Byrd, M. (1982). Aging and cognitive deficits: The role of attentional resources. In F. I. M. Craik & S. E. Trehub (Eds.), Aging and cognitive processes (pp. 191–211). Plenum Press. https://doi.org/10.1007/978-1-4684-4178-9_11
Chapter Google Scholar
Craik, F. I. M., & Salthouse, T. A. (2011). The Handbook of aging and cognition . Psychology Press.
Book Google Scholar
Diamond, A. (2013). Executive functions. Annual Review of Psychology, 64 , 135–168. https://doi.org/10.1146/annurev-psych-113011-143750
Donchin, E. (1981). Surprise!... Surprise? Psychophysiology, 18 (5), 493–513. https://doi.org/10.1111/j.1469-8986.1981.tb01815.x
Donchin, E., & Coles, M. G. H. (1988). Is the P300 component a manifestation of context updating? Behavioral and Brain Sciences, 11 (3), 357–374. https://doi.org/10.1017/s0140525x00058027
Dove, A., Pollmann, S., Schubert, T., Wiggins, C. J., & Yves von Cramon, D. (2000). Prefrontal cortex activation in task switching: an event-related fMRI study. Cognitive Brain Research, 9 (1), 103–109. https://doi.org/10.1016/S0926-6410(99)00029-4
Draheim, C., Hicks, K. L., & Engle, R. W. (2016). Combining reaction time and accuracy: The relationship between working memory capacity and task switching as a case example. Perspectives on Psychological Science, 11 (1), 133–155. https://doi.org/10.1177/1745691615596990
Dreisbach, G., & Fröber, K. (2019). On how to be flexible (or not): modulation of the stability-flexibility balance. Current Directions in Psychological Science, 28 (1), 3–9. https://doi.org/10.1177/0963721418800030
Eimer, M. (1993). Effects of attention and stimulus probability on ERPs in a Go/Nogo task. Biological Psychology, 35 (2), 123–138. https://doi.org/10.1016/0301-0511(93)90009-W
Engle, R. W. (2002). Working memory capacity as executive attention. Current Directions in Psychological Science, 11 (1), 19–23. https://doi.org/10.1111/1467-8721.00160
Esterman, M., & Rothlein, D. (2019). Models of sustained attention. Current Opinion in Psychology, 29 , 174–180. https://doi.org/10.1016/j.copsyc.2019.03.005
Filmer, H. L., Griffin, A., & Dux, P. E. (2019). For a minute there, I lost myself … dosage dependent increases in mind wandering via prefrontal tDCS. Neuropsychologia, 129 , 379–384. https://doi.org/10.1016/j.neuropsychologia.2019.04.013
Flannery, S. L., Jowett, T., Garvey, A., Cutfield, N. J., & Machado, L. (2018). Computerized testing in Parkinson’s disease: Performance deficits in relation to standard clinical measures. Journal of Clinical and Experimental Neuropsychology, 40 (10), 1062–1073. https://doi.org/10.1080/13803395.2018.1485880
Fortenbaugh, F. C., Rothlein, D., McGlinchey, R., DeGutis, J., & Esterman, M. (2018). Tracking behavioral and neural fluctuations during sustained attention: A robust replication and extension. NeuroImage, 171 , 148–164. https://doi.org/10.1016/j.neuroimage.2018.01.002
Foster, S. M., Cornwell, R. E., Kisley, M. A., & Davis, H. P. (2007). Cognitive changes across the lifespan. In S. H. Qualls & M. A. Smyer (Eds.), Changes in decision-making capacity in older adults: Assessment and intervention (pp. 25–60). Wiley.
Google Scholar
Fox, K. C. R., Spreng, R. N., Ellamil, M., Andrews-Hanna, J. R., & Christoff, K. (2015). The wandering brain: Meta-analysis of functional neuroimaging studies of mind-wandering and related spontaneous thought processes. NeuroImage, 111 , 611–621. https://doi.org/10.1016/j.neuroimage.2015.02.039
Franklin, M. S., Mrazek, M. D., Broadway, J. M., & Schooler, J. W. (2013). Disentangling decoupling: Comment on smallwood (2013). Psychological Bulletin, 139 (3), 536–541. https://doi.org/10.1037/a0030515
Frank, D. J., Nara, B., Zavagnin, M., Touron, D. R., & Kane, M. J. (2015). Validating older adults’ reports of less mind-wandering: An examination of eye movements and dispositional influences. Psychology and Aging, 30 (2), 266–278. https://doi.org/10.1037/pag0000031
Franklin, M. S., Mrazek, M. D., Anderson, C. L., Johnston, C., Smallwood, J., Kingstone, A., & Schooler, J. W. (2017). Tracking distraction: The relationship between mind- wandering, meta-awareness, and ADHD symptomatology. Journal of Attention Disorders, 21 (6), 475–486. https://doi.org/10.1177/1087054714543494
Fröber, K., & Dreisbach, G. (2017). Keep flexible–keep switching! The influence of forced task switching on voluntary task switching. Cognition, 162 , 48–53. https://doi.org/10.1016/j.cognition.2017.01.024
Fröber, K., Raith, L., & Dreisbach, G. (2018). The dynamic balance between cognitive flexibility and stability: The influence of local changes in reward expectation and global task context on voluntary switch rate. Psychological Research Psychologische Forschung, 82 (1), 65–77. https://doi.org/10.1007/s00426-017-0922-2
Gable, S. L., Hopper, E. A., & Schooler, J. W. (2019). When the muses strike: creative ideas of physicists and writers routinely occur during mind wandering. Psychological Science, 30 (3), 396–404. https://doi.org/10.1177/0956797618820626
Giambra, L. M. (1989). Task-unrelated thought frequency as a function of age: A laboratory study. Psychology and Aging, 4 (2), 136–143. https://doi.org/10.1037/0882-7974.4.2.136
Girn, M., Mills, C., Roseman, L., Carhart-Harris, R. L., & Christoff, K. (2020). Updating the dynamic framework of thought: creativity and psychedelics. NeuroImage, 213 , 116726. https://doi.org/10.1016/j.neuroimage.2020.116726
Guarino, A., Favieri, F., Boncompagni, I., Agostini, F., Cantone, M., & Casagrande, M. (2018). Executive functions in Alzheimer disease: A systematic review. Frontiers in Aging Neuroscience, 10 , 437. https://doi.org/10.3389/fnagi.2018.00437
Gyurkovics, M., Balota, D. A., & Jackson, J. D. (2018). Mind-wandering in healthy aging and early stage Alzheimer’s disease. Neuropsychology, 32 (1), 89–101. https://doi.org/10.1037/neu0000385
Halleland, H. B., Haavik, J., & Lundervold, A. J. (2012). Set-shifting in adults with ADHD. Journal of the International Neuropsychological Society, 18 (4), 728–737. https://doi.org/10.1017/S1355617712000355
Hommel, B. (2015). Between persistence and flexibility: The Yin and Yang of action control. In A. J. Elliot (Ed.), Advances in motivation science (Vol. 2, pp. 33–67). Elsevier.
Hommel, B., & Colzato, L. S. (2017). The social transmission of metacontrol policies: mechanisms underlying the interpersonal transfer of persistence and flexibility. Neuroscience and Biobehavioral Reviews, 81 , 43–58. https://doi.org/10.1016/j.neubiorev.2017.01.009
Hsieh, S., & Cheng, P. (2006). Task reconfiguration and carryover in task switching: an event-related potential study. Brain Research, 1084 (1), 132–145. https://doi.org/10.1016/j.brainres.2006.02.060
Huijser, S., van Vugt, M. K., & Taatgen, N. A. (2018). The wandering self: tracking distracting self-generated thought in a cognitively demanding context. Consciousness and Cognition, 58 , 170–185. https://doi.org/10.1016/j.concog.2017.12.004
Irons, J. L., & Leber, A. B. (2016). Choosing attentional control settings in a dynamically changing environment. Attention, Perception, & Psychophysics, 78 (7), 2031–2048. https://doi.org/10.3758/s13414-016-1125-4
Irwin, L. N., Kofler, M. J., Soto, E. F., & Groves, N. B. (2019). Do children with attention-deficit/hyperactivity disorder (ADHD) have set shifting deficits? Neuropsychology, 33 (4), 470–481. https://doi.org/10.1037/neu0000546
Jackson, J. D., & Balota, D. A. (2012). Mind-wandering in younger and older adults: converging evidence from the sustained attention to response task and reading for comprehension. Psychology and Aging, 27 (1), 106–119. https://doi.org/10.1037/a0023933
Jordão, M., Ferreira-Santos, F., Pinho, M. S., & St Jacques, P. L. (2019). Meta-analysis of aging effects in mind wandering: Methodological and sociodemographic factors. Psychology and Aging, 34 (4), 531–544. https://doi.org/10.1037/pag0000356
Jost, K., Mayr, U., & Rosler, F. (2008). Is task switching nothing but cue priming? Evidence from ERPs. Cognitive, Affective & Behavioral Neuroscience, 8 (1), 74–84. https://doi.org/10.3758/cabn.8.1.74
Kam, J. W., & Handy, T. C. (2014). Differential recruitment of executive resources during mind wandering. Consciousness and Cognition, 26 , 51–63. https://doi.org/10.1016/j.concog.2014.03.002
Kam, J. W. Y., Xu, J., & Handy, T. C. (2014). I don’t feel your pain (as much): The desensitizing effect of mind wandering on the perception of others’ discomfort. Cognitive, Affective, & Behavioral Neuroscience, 14 (1), 286–296. https://doi.org/10.3758/s13415-013-0197-z
Kane, M. J., Bleckley, M. K., Conway, A. R., & Engle, R. W. (2001). A controlled-attention view of working-memory capacity. Journal of Experimental Psychology: General, 130 (2), 169–183. https://doi.org/10.1037//0096-3445.130.2.169
Kane, M. J., Brown, L. H., McVay, J. C., Silvia, P. J., Myin-Germeys, I., & Kwapil, T. R. (2007). For whom the mind wanders, and when: an experience-sampling study of working memory and executive control in daily life. Psychological Science, 18 (7), 614–621. https://doi.org/10.1111/j.1467-9280.2007.01948.x
Kane, M. J., Smeekens, B. A., von Bastian, C. C., Lurquin, J. H., Carruth, N. P., & Miyake, A. (2017). A combined experimental and individual-differences investigation into mind wandering during a video lecture. Journal of Experimental Psychology: General, 146 (11), 1649–1674. https://doi.org/10.1037/xge0000362
Kasper, L. J., Alderson, R. M., & Hudec, K. L. (2012). Moderators of working memory deficits in children with attention-deficit/hyperactivity disorder (ADHD): A meta-analytic review. Clinical Psychology Review, 32 (7), 605–617. https://doi.org/10.1016/j.cpr.2012.07.001
Kessler, Y., Shencar, Y., & Meiran, N. (2009). Choosing to switch: Spontaneous task switching despite associated behavioral costs. Acta Psychologica, 131 (2), 120–128. https://doi.org/10.1016/j.actpsy.2009.03.005
Kieffaber, P. D., & Hetrick, W. P. (2005). Event-related potential correlates of task switching and switch costs. Psychophysiology, 42 (1), 56–71. https://doi.org/10.1111/j.1469-8986.2005.00262.x
Killingsworth, M. A., & Gilbert, D. T. (2010). A wandering mind is an unhappy mind. Science, 330 (6006), 932. https://doi.org/10.1126/science.1192439
Klinger, E. (1975). Consequences of commitment to and disengagement from incentives. Psychological Review, 82 (1), 1–25. https://doi.org/10.1037/h0076171
Klinger, E. (1999). Thought flow: Properties and mechanisms underlying shifts in content. In J. A. Singer & P. Salovey (Eds.), At play in the fields of consciousness: Essays in honor of Jerome L. Singer (pp. 29–50). Lawrence Erlbaum Associates Publishers.
Koch, I. (2003). The role of external cues for endogenous advance reconfiguration in task switching. Psychonomic Bulletin & Review, 10 (2), 488–492. https://doi.org/10.3758/bf03196511
Kok, A. (1986). Effects of degradation of visual stimuli on components of the event-related potential (ERP) in go/nogo reaction tasks. Biological Psychology, 23 (1), 21–38. https://doi.org/10.1016/0301-0511(86)90087-6
Kool, W., McGuire, J. T., Rosen, Z. B., & Botvinick, M. M. (2010). Decision making and the avoidance of cognitive demand. Journal of Experimental Psychology: General, 139 (4), 665–682. https://doi.org/10.1037/a0020198
Kramer, A. F., & Strayer, D. L. (1988). Assessing the development of automatic processing: An application of dual-task and event-related brain potential methodologies. Biological Psychology, 26 (1–3), 231–267. https://doi.org/10.1016/0301-0511(88)90022-1
Krawietz, S. A., Tamplin, A. K., & Radvansky, G. A. (2012). Aging and mind wandering during text comprehension. Psychology and Aging, 27 (4), 951–958. https://doi.org/10.1037/a0028831
Krimsky, M., Forster, D. E., Llabre, M. M., & Jha, A. P. (2017). The influence of time on task on mind wandering and visual working memory. Cognition, 169 , 84–90. https://doi.org/10.1016/j.cognition.2017.08.006
Kucyi, A., Salomons, T. V., & Davis, K. D. (2013). Mind wandering away from pain dynamically engages antinociceptive and default mode brain networks. Proceedings of the National Academy of Sciences, 110 (46), 18692–18697. https://doi.org/10.1073/pnas.1312902110
Kvavilashvili, L., & Rummel, J. (2020). On the nature of everyday prospection: a review and theoretical integration of research on mind-wandering, future thinking, and prospective memory. Review of General Psychology, 24 (3), 210–237. https://doi.org/10.1177/1089268020918843
Lau, H. C., & Passingham, R. E. (2007). Unconscious activation of the cognitive control system in the human prefrontal cortex. Journal of Neuroscience, 27 (21), 5805–5811. https://doi.org/10.1523/JNEUROSCI.4335-06.2007
Lindenberger, U., & Mayr, U. (2014). Cognitive aging: Is there a dark side to environmental support? Trends in Cognitive Sciences, 18 (1), 7–15. https://doi.org/10.1016/j.tics.2013.10.006
Lockenhoff, C. E., Rutt, J. L., Samanez-Larkin, G. R., Gallagher, C., O’Donoghue, T., & Reyna, V. F. (2020). Age effects in sequence construction for a continuous cognitive task: Similar sequence-trends but fewer switch-points. Journals of Gerontology: Series b, Psychological Sciences and Social Sciences, 75 (4), 762–771. https://doi.org/10.1093/geronb/gby090
Machado, L. (2021). Understanding cognition and how it changes with aging, brain disease, and lifestyle choices. Journal of the Royal Society of New Zealand, 51 (1), 128–142. https://doi.org/10.1080/03036758.2020.1796102
Maillet, D., & Rajah, M. N. (2013). Age-related changes in frequency of mind-wandering and task-related interferences during memory encoding and their impact on retrieval. Memory, 21 (7), 818–831. https://doi.org/10.1080/09658211.2012.761714
Maillet, D., & Rajah, M. N. (2016). Assessing the neural correlates of task-unrelated thoughts during episodic encoding and their association with subsequent memory in young and older adults. Journal of Cognitive Neuroscience, 28 (6), 826–841. https://doi.org/10.1162/jocn_a_00935
Maillet, D., & Schacter, D. L. (2016). From mind wandering to involuntary retrieval: age-related differences in spontaneous cognitive processes. Neuropsychologia, 80 , 142–156. https://doi.org/10.1016/j.neuropsychologia.2015.11.017
Maillet, D., Yu, L., Lau, B., Chow, R., Alain, C., & Grady, C. L. (2020). Differential effects of mind-wandering and visual distraction on age-related changes in neuro-electric brain activity and variability. Neuropsychologia, 146 , 107565. https://doi.org/10.1016/j.neuropsychologia.2020.107565
Martínez-Pérez, V., Baños, D., Andreu, A., Tortajada, M., Palmero, L. B., Campoy, G., & Fuentes, L. J. (2021). Propensity to intentional and unintentional mind-wandering differs in arousal and executive vigilance tasks. PLoS One, 16 (10), e0258734. https://doi.org/10.1371/journal.pone.0258734
Mason, M. F., Norton, M. I., Van Horn, J. D., Wegner, D. M., Grafton, S. T., & Macrae, C. N. (2007). Wandering minds: the default network and stimulus-independent thought. Science, 315 (5810), 393–395. https://doi.org/10.1126/science.1131295
Mazzoni, G. (2019). Involuntary memories and involuntary future thinking differently tax cognitive resources. Psychological Research Psychologische Forschung, 83 (4), 684–697. https://doi.org/10.1007/s00426-018-1123-3
McKinlay, A., Grace, R. C., Dalrymple-Alford, J. C., & Roger, D. (2010). Characteristics of executive function impairment in Parkinson’s disease patients without dementia. Journal of the International Neuropsychological Society, 16 (2), 268–277. https://doi.org/10.1017/S1355617709991299
McVay, J. C., & Kane, M. J. (2009). Conducting the train of thought: Working memory capacity, goal neglect, and mind wandering in an executive-control task. Journal of Experimental Psychology: Learning, Memory, and Cognition, 35 (1), 196–204. https://doi.org/10.1037/a0014104
McVay, J. C., & Kane, M. J. (2010). Does mind wandering reflect executive function or executive failure? Comment on Smallwood and Schooler (2006) and Watkins (2008). Psychological Bulletin, 136 (2), 188–197. https://doi.org/10.1037/a0018298
McVay, J. C., & Kane, M. J. (2012a). Drifting from slow to “D’oh!”: Working memory capacity and mind wandering predict extreme reaction times and executive control errors. Journal of Experimental Psychology: Learning, Memory, and Cognition, 38 (3), 525–549. https://doi.org/10.1037/a0025896
McVay, J. C., & Kane, M. J. (2012b). Why does working memory capacity predict variation in reading comprehension? On the influence of mind wandering and executive attention. Journal of Experimental Psychology: General, 141 (2), 302–320. https://doi.org/10.1037/a0025250
McVay, J. C., Kane, M. J., & Kwapil, T. R. (2009). Tracking the train of thought from the laboratory into everyday life: An experience-sampling study of mind wandering across controlled and ecological contexts. Psychonomic Bulletin & Review, 16 (5), 857–863. https://doi.org/10.3758/PBR.16.5.857
McVay, J. C., Meier, M. E., Touron, D. R., & Kane, M. J. (2013). Aging ebbs the flow of thought: adult age differences in mind wandering, executive control, and self-evaluation. Acta Psychologica, 142 (1), 136–147. https://doi.org/10.1016/j.actpsy.2012.11.006
Meiran, N., Chorev, Z., & Sapir, A. (2000). Component processes in task switching. Cognitive Psychology, 41 (3), 211–253. https://doi.org/10.1006/cogp.2000.0736
Miller, E. K., & Buschman, T. J. (2013). Cortical circuits for the control of attention. Current Opinion in Neurobiology, 23 (2), 216–222. https://doi.org/10.1016/j.conb.2012.11.011
Miller, E. K., & Cohen, J. D. (2001). An integrative theory of prefrontal cortex function. Annual Review of Neuroscience, 24 , 167–202. https://doi.org/10.1146/annurev.neuro.24.1.167
Mittelstädt, V., Miller, J., & Kiesel, A. (2019). Linking task selection to task performance: Internal and predictable external processing constraints jointly influence voluntary task switching behavior. Journal of Experimental Psychology: Human Perception and Performance, 45 (12), 1529–1548. https://doi.org/10.1037/xhp0000690
Miyake, A., Friedman, N. P., Emerson, M. J., Witzki, A. H., Howerter, A., & Wager, T. D. (2000). The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: A latent variable analysis. Cognitive Psychology, 41 (1), 49–100. https://doi.org/10.1006/cogp.1999.0734
Mooneyham, B. W., & Schooler, J. W. (2013). The costs and benefits of mind-wandering: A review. Canadian Journal of Experimental Psychology, 67 (1), 11–18. https://doi.org/10.1037/a0031569
Moran, C. N., McGovern, D. P., Warren, G., Grálaigh, R. Ó., Kenney, J. P. M., Smeaton, A., & Dockree, P. M. (2021). Young and restless, old and focused: Age-differences in mind-wandering frequency and phenomenology. Psychology and Aging, 36 (2), 252–267. https://doi.org/10.1037/pag0000526
Mowlem, F. D., Skirrow, C., Reid, P., Maltezos, S., Nijjar, S. K., Merwood, A., Barker, E., Cooper, R., Kuntsi, J., & Asherson, P. (2019). Validation of mind excessively wandering scale and the relationship of mind wandering to impairment in adult ADHD. Journal of Attention Disorders, 23 (6), 624–634. https://doi.org/10.1177/1087054716651927
Mueller, S. C., Swainson, R., & Jackson, G. M. (2007). Behavioural and neurophysiological correlates of bivalent and univalent responses during task switching. Brain Research, 1157 , 56–65. https://doi.org/10.1016/j.brainres.2007.04.046
Murray, S., & Krasich, K. (2020). Can the mind wander intentionally? Mind & Language . https://doi.org/10.1111/mila.12332
Murray, S., Liang, N., Brosowsky, N., & Seli, P. (2021). What are the benefits of mind wandering to creativity? Psychology of Aesthetics, Creativity, and the Arts . https://doi.org/10.1037/aca0000420
Nicosia, J., & Balota, D. (2021). Dispositional factors account for age differences in self-reported mind-wandering. Psychology and Aging, 36 (4), 421–432. https://doi.org/10.1037/pag0000614
Niedzwienska, A., & Kvavilashvili, L. (2018). Reduced mind-wandering in mild cognitive impairment: Testing the spontaneous retrieval deficit hypothesis. Neuropsychology, 32 (6), 711–723. https://doi.org/10.1037/neu0000457
Oberauer, K., Suss, H. M., Wilhelm, O., & Wittmann, W. W. (2003). The multiple faces of working memory: Storage, processing, supervision, and coordination. Intelligence, 31 (2), 167–193. https://doi.org/10.1016/S0160-2896(02)00115-0
Parks, C. W., Klinger, E., & Perlmutter, M. (1989). Dimensions of thought as a function of age, gender and task difficulty. Imagination, Cognition and Personality, 8 (1), 49–62. https://doi.org/10.2190/M6GA-J94F-VRV1-77DR
Patel, S. H., & Azzam, P. N. (2005). Characterization of N200 and P300: Selected studies of the event-related potential. International Journal of Medical Sciences, 2 (4), 147–154. https://doi.org/10.7150/ijms.2.147
Polich, J. (2007). Updating P300: an integrative theory of P3a and P3b. Clinical Neurophysiology, 118 (10), 2128–2148. https://doi.org/10.1016/j.clinph.2007.04.019
Poljac, E., & Yeung, N. (2014). Dissociable neural correlates of intention and action preparation in voluntary task switching. Cerebral Cortex, 24 (2), 465–478. https://doi.org/10.1093/cercor/bhs326
Posner, M. I., & Dehaene, S. (1994). Attentional networks. Trends in Neurosciences, 17 (2), 75–79. https://doi.org/10.1016/0166-2236(94)90078-7
Raichle, M. E., MacLeod, A. M., Snyder, A. Z., Powers, W. J., Gusnard, D. A., & Shulman, G. L. (2001). A default mode of brain function. Proceedings of the National Academy of Sciences, 98 (2), 676–682. https://doi.org/10.1073/pnas.98.2.676
Ralph, B. C. W., Thomson, D. R., Cheyne, J. A., & Smilek, D. (2014). Media multitasking and failures of attention in everyday life. Psychological Research Psychologische Forschung, 78 (5), 661–669. https://doi.org/10.1007/s00426-013-0523-7
Ramos, A. A., Hamdan, A. C., & Machado, L. (2020). A meta-analysis on verbal working memory in children and adolescents with ADHD. Clinical Neuropsychologist, 34 (5), 873–898. https://doi.org/10.1080/13854046.2019.1604998
Ramos, A. A., & Machado, L. (2021). A comprehensive meta-analysis on short-term and working memory dysfunction in Parkinson’s disease. Neuropsychology Review, 31 (2), 288–311. https://doi.org/10.1007/s11065-021-09480-w
Randall, J. G., Oswald, F. L., & Beier, M. E. (2014). Mind-wandering, cognition, and performance: A theory-driven meta-analysis of attention regulation. Psychological Bulletin, 140 (6), 1411–1431. https://doi.org/10.1037/a0037428
Ravizza, S. M., & Carter, C. S. (2008). Shifting set about task switching: behavioral and neural evidence for distinct forms of cognitive flexibility. Neuropsychologia, 46 (12), 2924–2935. https://doi.org/10.1016/j.neuropsychologia.2008.06.006
Reuss, H., Kiesel, A., Kunde, W., & Hommel, B. (2011). Unconscious activation of task sets. Consciousness and Cognition, 20 (3), 556–567. https://doi.org/10.1016/j.concog.2011.02.014
Rey-Mermet, A., & Gade, M. (2018). Inhibition in aging: What is preserved? What declines? A meta-analysis. Psychonomic Bulletin & Review, 25 (5), 1695–1716. https://doi.org/10.3758/s13423-017-1384-7
Robertson, I. H., Manly, T., Andrade, J., Baddeley, B. T., & Yiend, J. (1997). ‘Oops!’: performance correlates of everyday attentional failures in traumatic brain injured and normal subjects. Neuropsychologia, 35 (6), 747–758. https://doi.org/10.1016/s0028-3932(97)00015-8
Robison, M. K., & Unsworth, N. (2018). Cognitive and contextual correlates of spontaneous and deliberate mind-wandering. Journal of Experimental Psychology: Learning, Memory, and Cognition, 44 (1), 85–98. https://doi.org/10.1037/xlm0000444
Rohlf, H., Jucksch, V., Gawrilow, C., Huss, M., Hein, J., Lehmkuhl, U., & Salbach-Andrae, H. (2012). Set shifting and working memory in adults with attention-deficit/hyperactivity disorder. Journal of Neural Transmission, 119 (1), 95–106. https://doi.org/10.1007/s00702-011-0660-3
Rushworth, M. F., Passingham, R. E., & Nobre, A. C. (2002). Components of switching intentional set. Journal of Cognitive Neuroscience, 14 (8), 1139–1150. https://doi.org/10.1162/089892902760807159
Rushworth, M. F., Passingham, R. E., & Nobre, A. C. (2005). Components of attentional set-switching. Experimental Psychology, 52 (2), 83–98. https://doi.org/10.1027/1618-3169.52.2.83
Ryan, A. D., & Campbell, K. L. (2021). The ironic effect of older adults’ increased task motivation: Implications for neurocognitive aging. Psychonomic Bulletin & Review, 28 (6), 1743–1754. https://doi.org/10.3758/s13423-021-01963-4
Sakai, K., & Passingham, R. E. (2003). Prefrontal interactions reflect future task operations. Nature Neuroscience, 6 (1), 75–81. https://doi.org/10.1038/nn987
Schooler, J. W., Smallwood, J., Christoff, K., Handy, T. C., Reichle, E. D., & Sayette, M. A. (2011). Meta-awareness, perceptual decoupling and the wandering mind. Trends in Cognitive Sciences, 15 (7), 319–326. https://doi.org/10.1016/j.tics.2011.05.006
Seli, P., Smallwood, J., Cheyne, J. A., & Smilek, D. (2015). On the relation of mind wandering and ADHD symptomatology. Psychonomic Bulletin & Review, 22 (3), 629–636. https://doi.org/10.3758/s13423-014-0793-0
Seli, P., Risko, E. F., & Smilek, D. (2016a). On the necessity of distinguishing between unintentional and intentional mind wandering. Psychological Science, 27 (5), 685–691. https://doi.org/10.1177/0956797616634068
Seli, P., Risko, E. F., Smilek, D., & Schacter, D. L. (2016b). Mind-wandering with and without intention. Trends in Cognitive Sciences, 20 (8), 605–617. https://doi.org/10.1016/j.tics.2016.05.010
Seli, P., Maillet, D., Smilek, D., Oakman, J. M., & Schacter, D. L. (2017a). Cognitive aging and the distinction between intentional and unintentional mind wandering. Psychology and Aging, 32 (4), 315–324. https://doi.org/10.1037/pag0000172
Seli, P., Ralph, B. C. W., Konishi, M., Smilek, D., & Schacter, D. L. (2017b). What did you have in mind? Examining the content of intentional and unintentional types of mind wandering. Consciousness and Cognition, 51 , 149–156. https://doi.org/10.1016/j.concog.2017.03.007
Seli, P., O’Neill, K., Carriere, J. S. A., Smilek, D., Beaty, R. E., & Schacter, D. L. (2021). Mind-wandering across the age gap: age-related differences in mind-wandering are partially attributable to age-related differences in motivation. Journals of Gerontology: Series b, Psychological Sciences and Social Sciences, 76 (7), 1264–1271. https://doi.org/10.1093/geronb/gbaa031
Shaw, G. A., & Giambra, L. (1993). Task-unrelated thoughts of college students diagnosed as hyperactive in childhood. Developmental Neuropsychology, 9 (1), 17–30. https://doi.org/10.1080/87565649309540541
Shipstead, Z., Harrison, T. L., & Engle, R. W. (2015). Working memory capacity and the scope and control of attention. Attention, Perception, & Psychophysics, 77 (6), 1863–1880. https://doi.org/10.3758/s13414-015-0899-0
Smallwood, J. (2010). Why the global availability of mind wandering necessitates resource competition: reply to McVay and Kane (2010). Psychological Bulletin, 136 (2), 202–207. https://doi.org/10.1037/a0018673
Smallwood, J. (2013). Distinguishing how from why the mind wanders: A process-occurrence framework for self-generated mental activity. Psychological Bulletin, 139 (3), 519–535. https://doi.org/10.1037/a0030010
Smallwood, J., & Schooler, J. W. (2006). The restless mind. Psychological Bulletin, 132 (6), 946–958. https://doi.org/10.1037/0033-2909.132.6.946
Smallwood, J., Beach, E., Schooler, J. W., & Handy, T. C. (2008). Going AWOL in the brain: Mind wandering reduces cortical analysis of external events. Journal of Cognitive Neuroscience, 20 (3), 458–469. https://doi.org/10.1162/jocn.2008.20037
Smallwood, J., Nind, L., & O’Connor, R. C. (2009). When is your head at? An exploration of the factors associated with the temporal focus of the wandering mind. Consciousness and Cognition, 18 (1), 118–125. https://doi.org/10.1016/j.concog.2008.11.004
Smallwood, J., Brown, K. S., Tipper, C., Giesbrecht, B., Franklin, M. S., Mrazek, M. D., Carlson, J. M., & Schooler, J. W. (2011). Pupillometric evidence for the decoupling of attention from perceptual input during offline thought. PLoS One, 6 (3), e18298. https://doi.org/10.1371/journal.pone.0018298
Smallwood, J., Brown, K., Baird, B., & Schooler, J. W. (2012). Cooperation between the default mode network and the frontal–parietal network in the production of an internal train of thought. Brain Research, 1428 , 60–70. https://doi.org/10.1016/j.brainres.2011.03.072
Smallwood, J., Tipper, C., Brown, K., Baird, B., Engen, H., Michaels, J. R., Grafton, S., & Schooler, J. W. (2013). Escaping the here and now: Evidence for a role of the default mode network in perceptually decoupled thought. NeuroImage, 69 , 120–125. https://doi.org/10.1016/j.neuroimage.2012.12.012
Smallwood, J., & Schooler, J. W. (2015). The science of mind wandering: Empirically navigating the stream of consciousness. Annual Review of Psychology, 66 , 487–518. https://doi.org/10.1146/annurev-psych-010814-015331
Smallwood, J., Margulies, D., Bernhardt, B., & Jefferies, E. (2018). Investigating the elements of thought: Toward a component process account of spontaneous cognition. In K. Christoff & K. C. R. Fox (Eds.), The Oxford Handbook of spontaneous thought: Mind-wandering, creativity, and dreaming (pp. 71–83). Oxford University Press. https://doi.org/10.1093/oxfordhb/9780190464745.013.34
Sohn, M.-H., Ursu, S., Anderson, J. R., Stenger, V. A., & Carter, C. S. (2000). The role of prefrontal cortex and posterior parietal cortex in task switching. Proceedings of the National Academy of Sciences, 97 (24), 13448–13453. https://doi.org/10.1073/pnas.240460497
Stan, D., & Christoff, K. (2018). Potential clinical benefits and risks of spontaneous thought: Unconstrained attention as a way into and a way out of psychological disharmony. In K. Christoff & K. C. R. Fox (Eds.), The Oxford Handbook of spontaneous thought: Mind-wandering, creativity, and dreaming (pp. 479–491). Oxford University Press. https://doi.org/10.1093/oxfordhb/9780190464745.013.45
Stawarczyk, D., & D’Argembeau, A. (2015). Neural correlates of personal goal processing during episodic future thinking and mind-wandering: an ALE meta-analysis. Human Brain Mapping, 36 (8), 2928–2947. https://doi.org/10.1002/hbm.22818
Stawarczyk, D., Majerus, S., Maj, M., Van der Linden, M., & D’Argembeau, A. (2011). Mind-wandering: phenomenology and function as assessed with a novel experience sampling method. Acta Psychologica, 136 (3), 370–381. https://doi.org/10.1016/j.actpsy.2011.01.002
Steindorf, L., Hammerton, H. A., & Rummel, J. (2021). Mind wandering outside the box—about the role of off-task thoughts and their assessment during creative incubation. Psychology of Aesthetics, Creativity, and the Arts, 15 (4), 584–595. https://doi.org/10.1037/aca0000373
Taatgen, N. A., van Vugt, M. K., Daamen, J., Katidioti, I., Huijser, S., & Borst, J. P. (2021). The resource-availability model of distraction and mind-wandering. Cognitive Systems Research, 68 , 84–104. https://doi.org/10.1016/j.cogsys.2021.03.001
Terry, C. P., & Sliwinski, M. J. (2012). Aging and random task switching: the role of endogenous versus exogenous task selection. Experimental Aging Research, 38 (1), 87–109. https://doi.org/10.1080/0361073X.2012.637008
Thomson, D. R., Seli, P., Besner, D., & Smilek, D. (2014). On the link between mind wandering and task performance over time. Consciousness and Cognition, 27 , 14–26. https://doi.org/10.1016/j.concog.2014.04.001
Thomson, D. R., Besner, D., & Smilek, D. (2015). A resource-control account of sustained attention: evidence from mind-wandering and vigilance paradigms. Perspectives on Psychological Science, 10 (1), 82–96. https://doi.org/10.1177/1745691614556681
Tomasi, D., & Volkow, N. D. (2010). Functional connectivity density mapping. Proceedings of the National Academy of Sciences, 107 (21), 9885–9890. https://doi.org/10.1073/pnas.1001414107
Tomasi, D., & Volkow, N. D. (2012). Aging and functional brain networks. Molecular Psychiatry, 17 (5), 549–558. https://doi.org/10.1038/mp.2011.81
Traykov, L., Raoux, N., Latour, F., Gallo, L., Hanon, O., Baudic, S., Bayle, C., Wenisch, E., Remy, P., & Rigaud, A. S. (2007). Executive functions deficit in mild cognitive impairment. Cognitive and Behavioral Neurology, 20 (4), 219–224. https://doi.org/10.1097/WNN.0b013e31815e6254
Unsworth, N., & Robison, M. K. (2016). The influence of lapses of attention on working memory capacity. Memory & Cognition, 44 (2), 188–196. https://doi.org/10.3758/s13421-015-0560-0
Unsworth, N., & Robison, M. K. (2020). Working memory capacity and sustained attention: A cognitive-energetic perspective. Journal of Experimental Psychology: Learning, Memory, and Cognition, 46 (1), 77–103. https://doi.org/10.1037/xlm0000712
Vandamme, K., Szmalec, A., Liefooghe, B., & Vandierendonck, A. (2010). Are voluntary switches corrected repetitions? Psychophysiology, 47 (6), 1176–1181. https://doi.org/10.1111/j.1469-8986.2010.01032.x
Verleger, R. (2020). Effects of relevance and response frequency on P3b amplitudes: review of findings and comparison of hypotheses about the process reflected by P3b. Psychophysiology, 57 (7), e13542. https://doi.org/10.1111/psyp.13542
Vincent, J. L., Kahn, I., Snyder, A. Z., Raichle, M. E., & Buckner, R. L. (2008). Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. Journal of Neurophysiology, 100 (6), 3328–3342. https://doi.org/10.1152/jn.90355.2008
Voss, M. J., Zukosky, M., & Wang, R. F. (2018). A new approach to differentiate states of mind wandering: effects of working memory capacity. Cognition, 179 , 202–212. https://doi.org/10.1016/j.cognition.2018.05.013
Walpola, I. C., Muller, A. J., Hall, J. M., Andrews-Hanna, J. R., Irish, M., Lewis, S. J. G., Shine, J. M., & O’Callaghan, C. (2020). Mind-wandering in Parkinson’s disease hallucinations reflects primary visual and default network coupling. Cortex, 125 , 233–245. https://doi.org/10.1016/j.cortex.2019.12.023
Wasylyshyn, C., Verhaeghen, P., & Sliwinski, M. J. (2011). Aging and task switching: A meta-analysis. Psychology and Aging, 26 (1), 15–20. https://doi.org/10.1037/a0020912
Weibel, S., Giersch, A., Dehaene, S., & Huron, C. (2013). Unconscious task set priming with phonological and semantic tasks. Consciousness and Cognition, 22 (2), 517–527. https://doi.org/10.1016/j.concog.2013.02.010
Welz, A., Reinhard, I., Alpers, G. W., & Kuehner, C. (2018). Happy thoughts: Mind wandering affects mood in daily life. Mindfulness, 9 (1), 332–343. https://doi.org/10.1007/s12671-017-0778-y
Wickens, C., Kramer, A., Vanasse, L., & Donchin, E. (1983). Performance of concurrent tasks: A psychophysiological analysis of the reciprocity of information-processing resources. Science, 221 (4615), 1080–1082. https://doi.org/10.1126/science.6879207
Willcutt, E. G., Doyle, A. E., Nigg, J. T., Faraone, S. V., & Pennington, B. F. (2005). Validity of the executive function theory of attention-deficit/hyperactivity disorder: A meta-analytic review. Biological Psychiatry, 57 (11), 1336–1346. https://doi.org/10.1016/j.biopsych.2005.02.006
Wiradhany, W., & Koerts, J. (2021). Everyday functioning-related cognitive correlates of media multitasking: A mini meta-analysis. Media Psychology, 24 (2), 276–303. https://doi.org/10.1080/15213269.2019.1685393
Wirth, R., Foerster, A., Herbort, O., Kunde, W., & Pfister, R. (2018). This is how to be a rule breaker. Advances in Cognitive Psychology, 14 (1), 21–37. https://doi.org/10.5709/acp-0235-2
Wylie, G. R., Javitt, D. C., & Foxe, J. J. (2003). Task switching: A high-density electrical mapping study. NeuroImage, 20 (4), 2322–2342. https://doi.org/10.1016/j.neuroimage.2003.08.010
Yamaoka, A., & Yukawa, S. (2020). Does mind wandering during the thought incubation period improve creativity and worsen mood? Psychological Reports, 123 (5), 1785–1800. https://doi.org/10.1177/0033294119896039
Yanko, M. R., & Spalek, T. M. (2014). Driving with the wandering mind: The effect that mind-wandering has on driving performance. Human Factors, 56 (2), 260–269. https://doi.org/10.1177/0018720813495280
Zamani, A., Carhart-Harris, R., & Christoff, K. (2022). Prefrontal contributions to the stability and variability of thought and conscious experience. Neuropsychopharmacology, 47 (1), 329–348. https://doi.org/10.1038/s41386-021-01147-7
Zavagnin, M., Borella, E., & De Beni, R. (2014). When the mind wanders: Age-related differences between young and older adults. Acta Psychologica, 145 , 54–64. https://doi.org/10.1016/j.actpsy.2013.10.016
Zhang, W., Sjoerds, Z., & Hommel, B. (2020). Metacontrol of human creativity: The neurocognitive mechanisms of convergent and divergent thinking. NeuroImage, 210 , 116572. https://doi.org/10.1016/j.neuroimage.2020.116572
Zhuo, B., Zhu, M., Cao, B., & Li, F. (2021). More change in task repetition, less cost in task switching: Behavioral and event-related potential evidence. European Journal of Neuroscience, 53 (8), 2553–2566. https://doi.org/10.1111/ejn.15113
Zukosky, M., & Wang, R. F. (2021). Spontaneous state alternations in the time course of mind wandering. Cognition, 212 , 104689. https://doi.org/10.1016/j.cognition.2021.104689
Download references
Acknowledgements
Y. - S.W. acknowledges the receipt of a PhD scholarship from the University of Otago. We thank Dr Marijn Kouwenhoven and Wayne Meighan for comments on earlier drafts. We have no known conflict of interest to disclose.
Open Access funding enabled and organized by CAUL and its Member Institutions. Y.-S.W. acknowledges the receipt of a PhD scholarship from the University of Otago. The authors did not receive support from any organization for the submitted work.
Author information
Authors and affiliations.
Department of Psychology and Brain Health Research Centre, University of Otago, William James Building, 275 Leith Walk, Dunedin, 9016, New Zealand
Yi-Sheng Wong & Liana Machado
Brain Research New Zealand, Auckland, New Zealand
School of Psychology and Clinical Language Sciences, University of Reading Malaysia, Nusajaya, Malaysia
Yi-Sheng Wong & Adrian R. Willoughby
Department of Psychology, Monash University Malaysia, Subang Jaya, Malaysia
Adrian R. Willoughby
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Yi-Sheng Wong .
Ethics declarations
Conflict of interest.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval
Consent to participate, consent for publication, additional information, publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Wong, YS., Willoughby, A. & Machado, L. Reconceptualizing mind wandering from a switching perspective. Psychological Research 87 , 357–372 (2023). https://doi.org/10.1007/s00426-022-01676-w
Download citation
Received : 13 September 2021
Accepted : 10 March 2022
Published : 29 March 2022
Issue Date : March 2023
DOI : https://doi.org/10.1007/s00426-022-01676-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Find a journal
- Publish with us
- Track your research
Visual perspective and the characteristics of mind wandering
Affiliation.
- 1 School of Psychology, University of Aberdeen Aberdeen, UK.
- PMID: 24130538
- PMCID: PMC3793122
- DOI: 10.3389/fpsyg.2013.00699
When the mind wanders away from the here-and-now toward imaginary events, it typically does so from one of two visual vantage points-a first-person perspective (i.e., the world is seen as it is in everyday life) or a third-person perspective (i.e., the world is seen from the viewpoint of an outside observer). While extant evidence has detailed consequences that ensue from the utilization of these distinct points of view, less is known about their more basic properties. Here, we investigated the prevalence, demographics and qualities associated with the visual perspective that people spontaneously adopt when the mind wanders. The results from a cross-cultural survey (N = 400) revealed that almost half of the participants (46%) typically utilize a third-person perspective when mind wandering. Further, culture and gender were shown to impact the distribution of first- and third-person imagers. Specifically, a first-person perspective was more common among participants from Western nations and females, while participants from Eastern cultures resonated more strongly with a third-person perspective. Moreover, these factors were also shown to impact qualities (e.g., temporal locus, vividness) of mental imagery. Taken together, the current findings elucidate the prevalence of first- and third-person visual perspectives and detail individual differences that influence the qualia of mind wandering.
Keywords: cross cultural; mental imagery; mind wandering; third person; vantage point; visual perspective.
Researchers target neurogenesis in new approach to treat Parkinson's disease
Researchers at the University of Toronto have found a way to better control the preclinical generation of key neurons depleted in Parkinson's disease, pointing toward a new approach for a disease with no cure and few effective treatments.
The researchers used an antibody to selectively activate a receptor in a molecular signaling pathway to develop dopaminergic neurons. These neurons produce dopamine, a neurotransmitter critical to brain health.
Researchers around the world have been working to coax stem cells to differentiate into dopaminergic neurons, to replace those lost in patients living with Parkinson's disease. But efforts have been hindered in part by an inability to target specific receptors and areas of the brain.
"We used synthetic antibodies that we had previously developed to target the Wnt signaling pathway," said Stephane Angers, principal investigator on the study and director of the Donnelly Centre for Cellular and Molecular Biology.
"We can selectively activate this pathway to direct stem cells in the midbrain to develop into neurons by targeting specific receptors in the pathway," said Angers, who is also a professor in the Leslie Dan Faculty of Pharmacy and the Temerty Faculty of Medicine, and holds the Charles H. Best Chair of Medical Research at U of T. "This activation method has not been explored before."
The study was recently published in the journal Development .
Parkinson's disease is the second-most common neurological disorder after Alzheimer's, affecting over 100,000 Canadians. It particularly impacts older men, progressively impairing movement and causing pain as well as sleep and mental health issues.
Most previous research efforts to activate the Wnt signaling pathway have relied on a GSK3 enzyme inhibitor. This method involves multiple signaling pathways for stem cell proliferation and differentiation, which can lead to unintended effects on the newly produced neurons and activation of off-target cells.
"We developed an efficient method for stimulating stem cell differentiation to produce neural cells in the midbrain," said Andy Yang, first author on the study and a PhD student at the Donnelly Centre. "Moreover, cells activated via the FZD5 receptor closely resemble dopaminergic neurons of natural origin."
Another promising finding of the study was that implanting the artificially-produced neurons in a rodent model with Parkinson's disease led to improvement of the rodent's locomotive impairment.
"Our next step would be to continue using rodent or other suitable models to compare the outcomes of activating the FZD5 receptor and inhibiting GSK3," said Yang. "These experiments will confirm which method is more effective in improving symptoms of Parkinson's disease ahead of clinical trials."
This research was supported by the University of Toronto Medicine by Design program, which receives funding from the Canada First Research Excellence Fund, and the Canadian Institutes of Health Research.
- Parkinson's Research
- Diseases and Conditions
- Chronic Illness
- Disorders and Syndromes
- Parkinson's
- Alzheimer's
- Stem cell treatments
- Parkinson's disease
- Excitotoxicity and cell damage
- Biological psychiatry
- Deep brain stimulation
- Huntington's disease
- Multiple sclerosis
- Infectious disease
Story Source:
Materials provided by University of Toronto . Original written by Anika Hazra. Note: Content may be edited for style and length.
Journal Reference :
- Andy Yang, Rony Chidiac, Emma Russo, Hendrik Steenland, Quinn Pauli, Robert Bonin, Levi L. Blazer, Jarrett J. Adams, Sachdev S. Sidhu, Aleksandrina Goeva, Ali Salahpour, Stephane Angers. Exploiting spatiotemporal regulation of FZD5 during neural patterning for efficient ventral midbrain specification . Development , 2024; 151 (5) DOI: 10.1242/dev.202545
Cite This Page :
Explore More
- Fastest Rate of CO2 Rise Over Last 50,000 Years
- Like Dad and Like Mum...all in One Plant
- What Makes a Memory? Did Your Brain Work Hard?
- Plant Virus Treatment for Metastatic Cancers
- Controlling Shape-Shifting Soft Robots
- Brain Flexibility for a Complex World
- ONe Nova to Rule Them All
- AI Systems Are Skilled at Manipulating Humans
- Planet Glows With Molten Lava
- A Fragment of Human Brain, Mapped
Trending Topics
Strange & offbeat.
How alternative, holistic therapies can help those with neurodegenerative disorders like Parkinson's
When Beth Elgort’s husband, Steve, was diagnosed with Parkinson’s disease around a dozen years ago, she made it her mission to optimize his quality of life while the couple adjusted to their new normal.
Believing that South Florida’s tropical climate would be more conducive to helping him be active, the couple relocated from New York to Palm Beach Gardens.
In addition to seeking the opinions of medical professionals, Beth, 67 — a longtime mental health counselor, social worker and nutrition expert — also did copious research on her own about alternative and holistic therapies.
The Elgorts found that by keeping Steve, 77, physically active and creatively engaged, the worst symptoms of the neurodegenerative disease could be somewhat mitigated.
More local research: FIU teaching computers to diagnose strokes based on data from thousands of patients
More Health Matters: The McDonald's diet? How one man has lost 40-plus pounds eating only fast food
Health priorities: We're in this together: Fighting the loneliness epidemic
And their success in combatting Steve’s disease progression led the couple to want to share what they’d learned with others who were living with Parkinson’s.
“After years of social work practice, it was only natural to be a resource for my husband,” Beth Elgort said. “This grew into a desire to also advocate for others who have neurodegenerative diseases. My dream was to create a center that is a nurturing, safe environment where people can socialize and feel comfortable while taking classes to improve their neuroplasticity and movement.”
From helping husband with Parkinson's to helping many
So, in February 2020, just before the world went into pandemic lockdown, Beth realized a years-long dream: She founded the nonprofit Mind, Music and Movement Foundation for Neurological Disorders.
During that first year, most of the entity’s offerings were virtual. But as time passed and restrictions eased, they were able to begin offering in-person programming at Tropical Sands Christian Church (2726 Burns Road in Palm Beach Gardens).
Among the offerings for Mind, Music and Movement clients:
- The Voices of Parkinson’s Chorus
- Yoga and meditation
- Dance for fluidity
- Nutritional counseling
- Support services
West Palm Beach neurologist Dr. Arif Dalvi, director of the Comprehensive Movement Disorders Program at Palm Beach Neuroscience Institute and a member of Mind, Music and Movement’s medical advisory committee, is a proponent of taking a multi-pronged approach to attacking neurodegenerative diseases.
“Neurological disorders like Parkinson's disease are complex conditions that develop due to the death of the brain cells that produce dopamine,” Dalvi said.
When medications are needed and potentially effective, Dalvi will certainly prescribe them. But he’s quick to add that “for someone with a neurological disorder, a holistic approach is needed — one that combines attention to diet, lifestyle, and supportive therapies, including physical and speech therapy.”
Collaboration with Florida Atlantic University
As effective as Mind, Music and Movement has been at providing the community’s neurodegenerative disease sufferers with classes and advocacy, Beth wanted to reach even more people.
So last month the organization announced a new partnership with Florida Atlantic University’s Stiles-Nicholson Brain Institute and Schmidt College of Medicine.
“I have seen, firsthand, how alternative therapies help people thrive and possibly delay the progression of neurodegenerative diseases,” Elgort said. “This next step with FAU will help us reach even more people with our programming and events. And we are excited to participate in the Palm Beach County NeuroArts Collaborative with Palm Health Foundation and others.”
Elgort explained that plans for the collaboration between her organization and FAU include integrating alternative therapy programs into FAU’s Schmidt College of Medicine to further provide resources and motivation for people to live well with neurodegenerative diseases. Early programming will include FAU High School STEM students volunteering community service hours to assist with their daily classes. The clients participating will also have access to be part of current research studies related to neurodegenerative diseases at FAU.
“We are excited to partner with Mind, Music and Movement to expand the programs and services we provide to our students and our community to include the arts such as dance and music to enhance quality of life for those with Parkinson’s Disease,“ said Dr. Julie G. Pilitsis, dean of the Charles E. Schmidt College of Medicine and vice president for Medical Affairs at FAU. “This collaboration will help us to advance evidence-based clinical studies on alternative methods to improve movement, mood and cognition in those affected by neurodegenerative disorders, especially Parkinson’s disease.”
“Our partnership with Mind, Music and Movement will enable us to combine our expertise and resources to provide our community with the latest advances and tools for brain health and will help to propel the arts in neuroscience in this region,” noted Dr. Randy D. Blakely, neuroscience professor of biomedical science in the FAU Schmidt College of Medicine.
“In particular, the programs will provide an opportunity for faculty and trainees of the Institute’s David and Lynn Nicholson Center for Neurodegenerative Disease Research to engage closely with members of the community whose disorders they’re striving to understand and ultimately cure,” Blakely said.
Mind, Music and Movement offers a variety of pricing options for its offerings (some of which are virtual) — ranging from an unlimited monthly membership to group bundles to individual classes.
And for those who simply cannot afford the classes, Beth says “we believe wellness should be accessible to everyone no matter their financial status. If you’re unable to pay for classes, please contact us anyway because you may qualify for a scholarship.”
To learn more about Mind, Music and Movement Foundation for Neurological Disorders, call 561-336-0902 or visit m3f.org .

IMAGES
VIDEO
COMMENTS
Visual hallucinations are an underappreciated symptom affecting the majority of patients during the natural history of Parkinson's disease. Little is known about other forms of abstract and internally generated cognition - such as mind-wandering - in this population, but emerging evidence suggests that an interplay between the brain's primary visual and default networks might play a ...
Behavioural Neurology Mind-wandering in Parkinson's disease hallucinations reflects primary visual and default network coupling Ishan C. Walpola a, Alana J. Muller a, Julie M. Hall a,b, Jessica R. Andrews-Hanna c, Muireann Irish a,d,e, Simon J.G. Lewis a, James M. Shine a and Claire O'Callaghan a,f,* a Brain and Mind Centre and Central Clinical School, Faculty of Medicine and Health ...
Walpola, I. C. et al. Mind-wandering in Parkinson's disease hallucinations reflects primary visual and default network coupling. Cortex 125 , 233-245 (2020). Article PubMed Google Scholar
Recently, reduced recruitment and connectivity of the DMN has been described in Parkinson's disease (PD) patients compared to healthy controls. We thus aimed to explore whether PD patients with normal cognitive test scores show differential MW capabilities compared to healthy controls. Methods: Thirty PD patients and thirty age-matched controls ...
Mind-wandering in Parkinson's disease hallucinations reflects primary visual and default network coupling Ishan C. Walpola, Alana J. Muller, Julie M. Hall, Jessica R. Andrews-Hanna , Muireann Irish, Simon J.G. Lewis, James M. Shine, Claire O'Callaghan
As the frequency of mind-wandering in Parkinson's disease with hallucination was not significantly different than in the control group, whereas controls reported a significantly higher number of ...
To address this, we administered a validated thought-sampling task to 38 Parkinson's disease patients (18 with hallucinations; 20 without) and 40 controls, to test the hypothesis that individuals with hallucinations experience an increased frequency of mind-wandering - a form of spontaneous cognition strongly associated with mental imagery ...
Mind-wandering in Parkinson's disease hallucinations reflects primary visual and default network coupling @article{Walpola2020MindwanderingIP, title={Mind-wandering in Parkinson's disease hallucinations reflects primary visual and default network coupling}, author={Ishan C. Walpola and Alana J. Muller and Julie M. Hall and Jessica R. Andrews ...
Background: Mind Wandering (MW) refers to the process of disengaging from the immediate external environment and participating in internally driven mentation. This process has been suggested to be supported by a distributed set of brain regions, collectively referred to as the Default Mode Network (DMN). Recently, reduced recruitment and connectivity of the DMN has been described in Parkinson ...
Visual hallucinations are an underappreciated symptom affecting the majority of patients during the natural history of Parkinson's disease. Little is known about other forms of abstract and internally generated cognition - such as mind-wandering - in this population, but emerging evidence suggests that an interplay between the brain's primary visual and default networks might play a crucial ...
The DMN is associated with internally focused tasks, e.g. mind wandering, whereas the VAN is used for salience monitoring and mediates the switch between the DAN and the DMN. The attentional networks hypothesis predicts that visual hallucinations in Parkinson's disease are caused by overactivity of the DMN and VAN reinforcing false images ...
Patients with visual hallucinations had higher mind-wandering scores, and degree of mind-wandering was associated with stronger functional connectivity between the primary visual cortex and the default mode network. Hepp et al. assessed functional connectivity using synchronisation likelihood in 15 PD-VH. They found reduced functional ...
Cognitive Changes. Some people with Parkinson's disease (PD) experience mild cognitive impairment. Feelings of distraction or disorganization can accompany cognitive impairment, along with finding it difficult to plan and accomplish tasks. It may be harder to focus in situations that divide your attention, like a group conversation.
Using this task, we measured mind-wandering frequencies in Parkinson's disease patients with and without visual hallucinations, and healthy controls. To explore the neural correlates of mind-wandering frequency, we used network-level and seed-to-voxel analysis of resting-state functional magnetic resonance imaging. We were
Moments when our minds wander can allow space for creativity and planning for the future, he says, so it makes sense that many parts of the brain would be engaged in that kind of thinking. But mind wandering may also be detrimental, especially for those suffering from mental illness, explains the study's senior author, Susan Whitfield ...
Here, we explored the association between mind-wandering and visual hallucinations in Parkinson's disease, and their relationship with brain network coupling. We administered a validated thought-sampling task to 38 Parkinson's disease patients (18 with hallucinations; 20 without) and 40 controls, to test the hypothesis that individuals with ...
In Parkinson's disease (PD) with visual hallucinations, over-activity in the default mode network (DMN) is a hypothesised source of excessive top-down ... hallucinators experienced increased mind-wandering - a form of spontaneous thought strongly associated with DMN activity. Neural correlates of the behavioural
Mind-wandering increases in frequency over time during task performance: An individual-participant meta-analytic review ... AI, robotics, neurology, brain cancer, mental health, machine learning, autism, Parkinson's, Alzheimer's, brain research, depression and other sciences. Leave a Reply Cancel reply. Your email address will not be published ...
Mind wandering is a universal phenomenon in which our attention shifts away from the task at hand toward task-unrelated thoughts. Despite it inherently involving a shift in mental set, little is known about the role of cognitive flexibility in mind wandering. In this article we consider the potential of cognitive flexibility as a mechanism for mediating and/or regulating the occurrence of mind ...
Visual hallucinations are an underappreciated symptom affecting the majority of patients during the natural history of Parkinson's disease.Little is known about other forms of abstract and internally generated cognition - such as mind-wandering - in this population, but emerging evidence suggests that an interplay between the brain's primary visual and default networks might play a crucial ...
Mind-wandering has a controversial relationship with cognitive control. Existing psychological evidence supports the hypothesis that episodes of mind-wandering reflect a failure to constrain thinking to task-relevant material, as well the apparently alternative view that control can facilitate the expression of self-generated mental content.
Here, we investigated the prevalence, demographics and qualities associated with the visual perspective that people spontaneously adopt when the mind wanders. The results from a cross-cultural survey (N = 400) revealed that almost half of the participants (46%) typically utilize a third-person perspective when mind wandering. Further, culture ...
Doctors prescribe antidepressants to treat depression as well as other conditions. Antidepressant use can cause drug-induced parkinsonism in some people. They may also cause side effects such as ...
Researchers have found a way to better control the preclinical generation of key neurons depleted in Parkinson's disease, pointing toward a new approach for a disease with no cure and few ...
To learn more about Mind, Music and Movement Foundation for Neurological Disorders, call 561-336-0902 or visit m3f.org. A Palm Beach County nonprofit teams with FAU to hone holistic approach to ...