- Type 2 Diabetes
- Heart Disease
- Digestive Health
- Multiple Sclerosis
- Diet & Nutrition
- Supplements
- Health Insurance
- Public Health
- Patient Rights
- Caregivers & Loved Ones
- End of Life Concerns
- Health News
- Thyroid Test Analyzer
- Doctor Discussion Guides
- Hemoglobin A1c Test Analyzer
- Lipid Test Analyzer
- Complete Blood Count (CBC) Analyzer
- What to Buy
- Editorial Process
- Meet Our Medical Expert Board
What Is ATP?
Adenosine triphosphate (ATP) is every living cell's source of energy
- How It Works
- How It's Made
- Why It's So Important
Frequently Asked Questions
Adenosine triphosphate (ATP) is an energy-carrying molecule known as "the energy currency of life" or "the fuel of life," because it's the universal energy source for all living cells.
Every living organism consists of cells that rely on ATP for their energy needs. ATP is made by converting the food we eat into energy. It's an essential building block for all life forms. Without ATP, cells wouldn't have the fuel or power to perform functions necessary to stay alive, and they would eventually die. All forms of life rely on ATP to do the things they must do to survive.
This article explains how adenosine triphosphate works, how it's made, why ATP is so important to cellular processes, and what makes it vital to all life forms.
Verywell / Getty Images
How ATP Works
ATP is made of a nitrogen base (adenine) and a sugar molecule (ribose), which create adenosine, plus three phosphate molecules. If adenosine only has one phosphate molecule, it’s called adenosine monophosphate (AMP). If it has two phosphates, it’s called adenosine diphosphate (ADP).
Although adenosine is a fundamental part of ATP, when it comes to providing energy to a cell and fueling cellular processes, the phosphate molecules are what really matter. The most energy-loaded composition for adenosine is ATP, which has three phosphates.
ATP was first discovered in the 1920s. In 1929, Karl Lohmann—a German chemist studying muscle contractions—isolated what we now call adenosine triphosphate in a laboratory. At the time, Lohmann called ATP by a different name. It wasn't until a decade later, in 1939, that Nobel Prize–winner Fritz Lipmann established that ATP is the universal carrier of energy in all living cells and coined the term "energy-rich phosphate bonds."
Lipmann focused on phosphate bonds as the key to ATP being the universal energy source for all living cells, because adenosine triphosphate releases energy when one of its three phosphate bonds breaks off to form ADP. ATP is a high-energy molecule with three phosphate bonds; ADP is low-energy with only two phosphate bonds.
The Twos and Threes of ATP and ADP
Adenosine tri phosphate (ATP) becomes adenosine di phosphate (ADP) when one of its three phosphate molecules breaks free and releases energy (“tri” means “three,” while “di” means “two”). Conversely, ADP becomes ATP when a phosphate molecule is added. As part of an ongoing energy cycle, ADP is constantly recycled back into ATP.
Much like a rechargeable battery with a fluctuating state of charge, ATP represents a fully charged battery, and ADP represents "low-power mode." Every time a fully charged ATP molecule loses a phosphate bond, it becomes ADP; energy is released via the process of ATP becoming ADP.
On the flip side, when a phosphate bond is added, ADP becomes ATP. When ADP becomes ATP, what was previously a low-charged energy adenosine molecule (ADP) becomes fully charged ATP. This energy-creation and energy-depletion cycle happens time and time again, much like your smartphone battery can be recharged countless times during its lifespan.
How ATP Is Made
The human body uses molecules held in the fats, proteins, and carbohydrates we eat or drink as sources of energy to make ATP. This happens through a process called hydrolysis .
After food is digested, it's synthesized into glucose, which is a form of sugar. Glucose is the main source of fuel that our cells' mitochondria use to convert caloric energy from food into ATP, which is an energy form that can be used by cells.
ATP is made via a process called cellular respiration that occurs in the mitochondria of a cell. Mitochondria are tiny subunits within a cell that specialize in extracting energy from the foods we eat and converting it into ATP.
Mitochondria can convert glucose into ATP via two different types of cellular respiration:
- Aerobic (with oxygen)
- Anaerobic (without oxygen)
Aerobic cellular respiration transforms glucose into ATP in a three-step process, as follows:
- Step 1: Glycolysis
- Step 2: The Krebs cycle (also called the citric acid cycle)
- Step 3: Electron transport chain
During glycolysis, glucose (i.e., sugar) from food sources is broken down into pyruvate molecules. This is followed by the Krebs cycle, which is an aerobic process that uses oxygen to finish breaking down sugar and harnesses energy into electron carriers that fuel the synthesis of ATP. Lastly, the electron transport chain (ETC) pumps positively-charged protons that drive ATP production throughout the mitochondria’s inner membrane.
Mitochondria Make ATP
Mitochondria are mini-structures within a cell that convert glucose into "the energy molecule" known as ATP via aerobic or anaerobic cellular respiration.
ATP can also be produced without oxygen (i.e., anaerobic), which is something plants, algae, and some bacteria do by converting the energy held in sunlight into energy that can be used by a cell via photosynthesis.
Anaerobic exercise means that your body is working out "without oxygen." Anaerobic glycolysis occurs in human cells when there isn't enough oxygen available during an anaerobic workout. If no oxygen is present during cellular respiration, pyruvate can't enter the Krebs cycle and is oxidized into lactic acid. In the absence of oxygen, lactic acid fermentation makes ATP anaerobically.
The burning sensation you feel in your muscles when you're huffing and puffing during anaerobic high-intensity interval training (HIIT) that maxes out your aerobic capacity or during a strenuous weight-lifting workout is lactic acid, which is used to make ATP via anaerobic glycolysis.
During aerobic exercise , mitochondria have enough oxygen to make ATP aerobically. However, when you're out of breath and your cells don’t have enough oxygen to perform cellular respiration aerobically, the process can still happen anaerobically, but it creates a temporary burning sensation in your skeletal muscles.
Why ATP Is So Important
ATP is essential for life and makes it possible for us to do the things we do. Without ATP, cells wouldn't be able to use the energy held in food to fuel cellular processes, and an organism couldn't stay alive.
As a real-world example, when a car runs out of gas and is parked on the side of the road, the only thing that will make the car drivable again is putting some gasoline back in the tank. For all living cells, ATP is like the gas in a car's fuel tank. Without ATP, cells wouldn't have a source of usable energy, and the organism would die.
A Word From Verywell
Eating a well-balanced diet and staying hydrated should give your body all the resources it needs to produce plenty of ATP. Although some athletes may slightly improve their performance by taking supplements or ergonomic aids designed to increase ATP production, it's debatable that oral adenosine triphosphate supplementation actually increases energy.
Always use common sense and talk to a healthcare provider before spending money or ingesting supplements that make potentially hyped-up marketing claims about increasing energy by boosting ATP production.
An average cell in the human body uses about 10 million ATP molecules per second and can recycle all of its ATP in less than a minute. Over 24 hours, the human body turns over its weight in ATP.
ATP deficiencies can reduce energy and make you feel lethargic. Although eating a well-balanced diet and staying hydrated should give your body enough fuel to produce plenty of ATP, certain diseases such as fibromyalgia and chronic fatigue syndrome may disrupt ATP hydrolysis.
Adenosine metabolism rates may affect your vulnerability to sleep deprivation and your deep-sleep quality. Research suggests that sleep-wake cycles are influenced by how adenosine is metabolized in the brain.
Morelli AM, Ravera S, Panfoli I. The aerobic mitochondrial ATP synthesis from a comprehensive point of view. Open Biol . 2020;10(10):200224. doi:10.1098/rsob.200224
Dunn J, Grider MH. Physiology, Adenosine Triphosphate. [Updated 2022 Feb 17]. In: StatPearls [Internet].
ScienceDirect Topics. Adenosine Triphosphate: Overview.
Max Planck Research. Cells flexing their muscle.
Surita G. The power of phosphate. Historical Studies in the Natural Sciences . 2022;52(1):1-39. doi:10.1525/hsns.2022.52.1.1
Hargreaves M, Spriet LL. Skeletal muscle energy metabolism during exercise. Nat Metab . 2020;2(9):817-828. doi:10.1038/s42255-020-0251-4
Morris G, Maes M. Mitochondrial dysfunctions in myalgic encephalomyelitis/chronic fatigue syndrome explained by activated immuno-inflammatory, oxidative and nitrosative stress pathways . Metab Brain Dis . 2014 Mar;29(1):19-36. doi:10.1007/s11011-013-9435-x
Mackiewicz M, Nikonova EV, Zimmerman JE, et al. Enzymes of adenosine metabolism in the brain: diurnal rhythm and the effect of sleep deprivation. J Neurochem . 2003;85(2):348-57. doi:10.1046/j.1471-4159.2003.01687.x
By Christopher Bergland Bergland is a retired ultra-endurance athlete turned medical writer and science reporter. He is based in Massachusetts.

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
Margin Size
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

5.4: Active Transport
- Last updated
- Save as PDF
- Page ID 75459

\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
Learning Objectives
By the end of this section, you will be able to:
- Understand how electrochemical gradients affect ions
- Distinguish between primary active transport and secondary active transport
Active transport mechanisms require the use of the cell’s energy, usually in the form of adenosine triphosphate (ATP). If a substance must move into the cell against its concentration gradient—that is, if the concentration of the substance inside the cell is greater than its concentration in the extracellular fluid (and vice versa)—the cell must use energy to move the substance. Some active transport mechanisms move small-molecular weight materials, such as ions, through the membrane. Other mechanisms transport much larger molecules.
Electrochemical Gradient
We have discussed simple concentration gradients—differential concentrations of a substance across a space or a membrane—but in living systems, gradients are more complex. Because ions move into and out of cells and because cells contain proteins that do not move across the membrane and are mostly negatively charged, there is also an electrical gradient, a difference of charge, across the plasma membrane. The interior of living cells is electrically negative with respect to the extracellular fluid in which they are bathed, and at the same time, cells have higher concentrations of potassium (K + ) and lower concentrations of sodium (Na + ) than does the extracellular fluid. So in a living cell, the concentration gradient of Na + tends to drive it into the cell, and the electrical gradient of Na + (a positive ion) also tends to drive it inward to the negatively charged interior. The situation is more complex, however, for other elements such as potassium. The electrical gradient of K + , a positive ion, also tends to drive it into the cell, but the concentration gradient of K + tends to drive K + out of the cell (Figure \(\PageIndex{1}\)). The combined gradient of concentration and electrical charge that affects an ion is called its electrochemical gradient .
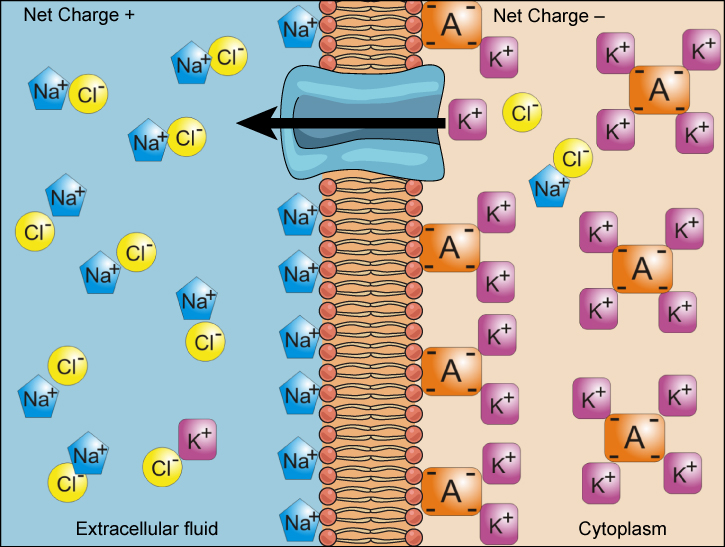
Moving Against a Gradient
To move substances against a concentration or electrochemical gradient, the cell must use energy. This energy is harvested from ATP generated through the cell’s metabolism. Active transport mechanisms, collectively called pumps , work against electrochemical gradients. Small substances constantly pass through plasma membranes. Active transport maintains concentrations of ions and other substances needed by living cells in the face of these passive movements. Much of a cell’s supply of metabolic energy may be spent maintaining these processes. (Most of a red blood cell’s metabolic energy is used to maintain the imbalance between exterior and interior sodium and potassium levels required by the cell.) Because active transport mechanisms depend on a cell’s metabolism for energy, they are sensitive to many metabolic poisons that interfere with the supply of ATP.
Two mechanisms exist for the transport of small-molecular weight material and small molecules. Primary active transport moves ions across a membrane and creates a difference in charge across that membrane, which is directly dependent on ATP. Secondary active transport describes the movement of material that is due to the electrochemical gradient established by primary active transport that does not directly require ATP.
Carrier Proteins for Active Transport
An important membrane adaption for active transport is the presence of specific carrier proteins or pumps to facilitate movement: there are three types of these proteins or transporters (Figure \(\PageIndex{2}\)). A uniporter carries one specific ion or molecule. A symporter carries two different ions or molecules, both in the same direction. An antiporter also carries two different ions or molecules, but in different directions. All of these transporters can also transport small, uncharged organic molecules like glucose. These three types of carrier proteins are also found in facilitated diffusion, but they do not require ATP to work in that process. Some examples of pumps for active transport are Na + -K + ATPase, which carries sodium and potassium ions, and H + -K + ATPase, which carries hydrogen and potassium ions. Both of these are antiporter carrier proteins. Two other carrier proteins are Ca 2+ ATPase and H + ATPase, which carry only calcium and only hydrogen ions, respectively. Both are pumps.
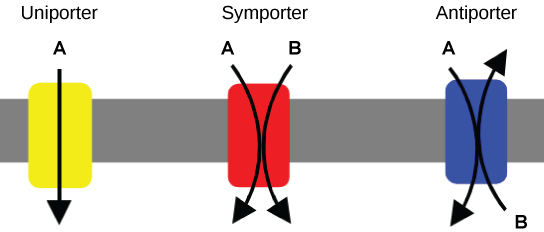
Primary Active Transport
The primary active transport that functions with the active transport of sodium and potassium allows secondary active transport to occur. The second transport method is still considered active because it depends on the use of energy as does primary transport (Figure \(\PageIndex{3}\)).
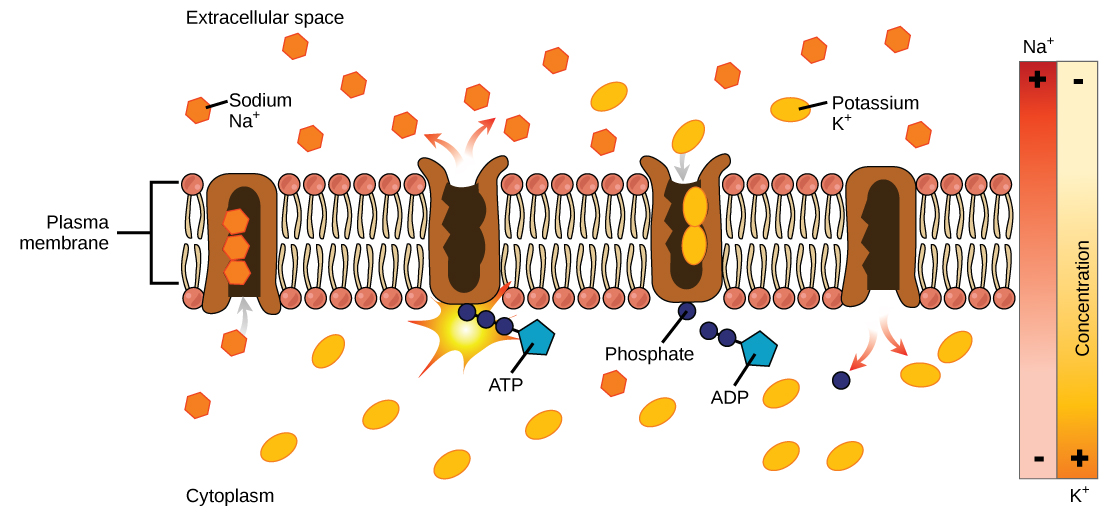
One of the most important pumps in animals cells is the sodium-potassium pump (Na + -K + ATPase), which maintains the electrochemical gradient (and the correct concentrations of Na + and K + ) in living cells. The sodium-potassium pump moves K + into the cell while moving Na + out at the same time, at a ratio of three Na + for every two K + ions moved in. The Na + -K + ATPase exists in two forms, depending on its orientation to the interior or exterior of the cell and its affinity for either sodium or potassium ions. The process consists of the following six steps.
- With the enzyme oriented towards the interior of the cell, the carrier has a high affinity for sodium ions. Three ions bind to the protein.
- ATP is hydrolyzed by the protein carrier and a low-energy phosphate group attaches to it.
- As a result, the carrier changes shape and re-orients itself towards the exterior of the membrane. The protein’s affinity for sodium decreases and the three sodium ions leave the carrier.
- The shape change increases the carrier’s affinity for potassium ions, and two such ions attach to the protein. Subsequently, the low-energy phosphate group detaches from the carrier.
- With the phosphate group removed and potassium ions attached, the carrier protein repositions itself towards the interior of the cell.
- The carrier protein, in its new configuration, has a decreased affinity for potassium, and the two ions are released into the cytoplasm. The protein now has a higher affinity for sodium ions, and the process starts again.
Several things have happened as a result of this process. At this point, there are more sodium ions outside of the cell than inside and more potassium ions inside than out. For every three ions of sodium that move out, two ions of potassium move in. This results in the interior being slightly more negative relative to the exterior. This difference in charge is important in creating the conditions necessary for the secondary process. The sodium-potassium pump is, therefore, an electrogenic pump (a pump that creates a charge imbalance), creating an electrical imbalance across the membrane and contributing to the membrane potential.
Interactive Link
View this video to see a simulation of active transport in a sodium-potassium ATPase.
Secondary Active Transport (Co-transport)
Secondary active transport brings sodium ions, and possibly other compounds, into the cell. As sodium ion concentrations build outside of the plasma membrane because of the action of the primary active transport process, an electrochemical gradient is created. If a channel protein exists and is open, the sodium ions will be pulled through the membrane. This movement is used to transport other substances that can attach themselves to the transport protein through the membrane (Figure \(\PageIndex{4}\)). Many amino acids, as well as glucose, enter a cell this way. This secondary process is also used to store high-energy hydrogen ions in the mitochondria of plant and animal cells for the production of ATP. The potential energy that accumulates in the stored hydrogen ions is translated into kinetic energy as the ions surge through the channel protein ATP synthase, and that energy is used to convert ADP into ATP.
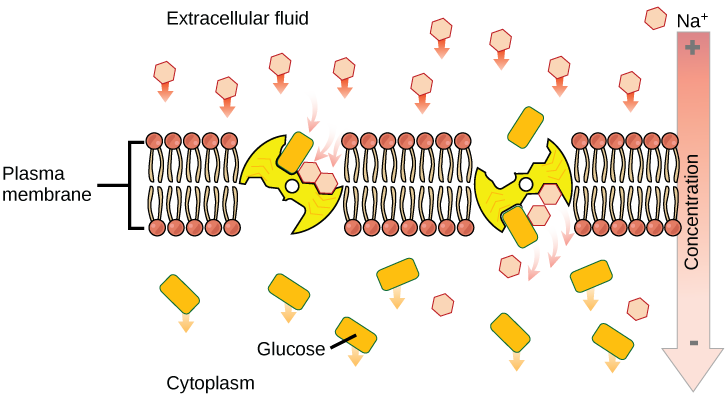
The combined gradient that affects an ion includes its concentration gradient and its electrical gradient. A positive ion, for example, might tend to diffuse into a new area, down its concentration gradient, but if it is diffusing into an area of net positive charge, its diffusion will be hampered by its electrical gradient. When dealing with ions in aqueous solutions, a combination of the electrochemical and concentration gradients, rather than just the concentration gradient alone, must be considered. Living cells need certain substances that exist inside the cell in concentrations greater than they exist in the extracellular space. Moving substances up their electrochemical gradients requires energy from the cell. Active transport uses energy stored in ATP to fuel this transport. Active transport of small molecular-sized materials uses integral proteins in the cell membrane to move the materials: These proteins are analogous to pumps. Some pumps, which carry out primary active transport, couple directly with ATP to drive their action. In co-transport (or secondary active transport), energy from primary transport can be used to move another substance into the cell and up its concentration gradient.
Review Questions
Active transport must function continuously because __________.
- plasma membranes wear out
- not all membranes are amphiphilic
- facilitated transport opposes active transport
- diffusion is constantly moving solutes in opposite directions
How does the sodium-potassium pump make the interior of the cell negatively charged?
- by expelling anions
- by pulling in anions
- by expelling more cations than are taken in
- by taking in and expelling an equal number of cations
What is the combination of an electrical gradient and a concentration gradient called?
- potential gradient
- electrical potential
- concentration potential
- electrochemical gradient
Free Response
Where does the cell get energy for active transport processes?
Answer: The cell harvests energy from ATP produced by its own metabolism to power active transport processes, such as the activity of pumps.
How does the sodium-potassium pump contribute to the net negative charge of the interior of the cell?
Answer: The sodium-potassium pump forces out three (positive) Na + ions for every two (positive) K + ions it pumps in, thus the cell loses a positive charge at every cycle of the pump.
Study Figure \(\PageIndex{1}\). Injection of a potassium solution into a person’s blood is lethal; this is used in capital punishment and euthanasia. Why do you think a potassium solution injection is lethal?
Answer: Cells typically have a high concentration of potassium in the cytoplasm and are bathed in a high concentration of sodium. Injection of potassium dissipates this electrochemical gradient. In heart muscle, the sodium/potassium potential is responsible for transmitting the signal that causes the muscle to contract. When this potential is dissipated, the signal can’t be transmitted, and the heart stops beating. Potassium injections are also used to stop the heart from beating during surgery.
Study Figure \(\PageIndex{4}\). If the pH outside the cell decreases, would you expect the amount of amino acids transported into the cell to increase or decrease?
Answer: A decrease in pH means an increase in positively charged H + ions, and an increase in the electrical gradient across the membrane. The transport of amino acids into the cell will increase.
Contributors and Attributions
Connie Rye (East Mississippi Community College), Robert Wise (University of Wisconsin, Oshkosh), Vladimir Jurukovski (Suffolk County Community College), Jean DeSaix (University of North Carolina at Chapel Hill), Jung Choi (Georgia Institute of Technology), Yael Avissar (Rhode Island College) among other contributing authors. Original content by OpenStax (CC BY 4.0; Download for free at http://cnx.org/contents/[email protected] ).

Physiology News Magazine

- Autumn 2007 - Issue Number 68
Circulating ATP and ADP: important regulators of blood flow and platelet reactivity during exercise
Gennady G Yegutkin (1) & José González-Alonso (2)
1: MediCity Research Laboratory, University of Turku and National Public Health Institute, Turku, Finland 2: Centre for Sports Medicine and Human Performance, Brunel University, Uxbridge, UK
https://doi.org/10.36866/pn.68.31

Extracellular ATP and other nucleotides (ADP, UTP, UDP) are important signalling molecules in the cardiovascular system, where they induce diverse vasodilatatory, immunomodulatory and prothrombotic responses (Bours et al. 2006; Burnstock, 2006). These effects are mediated through G-protein-coupled P2Y receptors as well as via ligand-gated P2X receptors (Fig.1 A ), which are ubiquitously expressed on various cell types, including the vascular endothelium and haematopoietic cells. Subsequent to signal transduction, nucleotides need to be rapidly inactivated and vascular endothelial ectoenzymes nucleoside triphosphate diphosphohydrolase (NTPDase; known as ecto-ATPDase, CD39) and ecto-5’-nucleotidase/CD73 are considered the major regulators of the duration and magnitude of purinergic signalling in the vasculature (Bours et al. 2006). In contrast to traditional paradigms that focus on nucleotide-inactivating mechanisms, it has now become clear that ‘classical’ intracellular enzymes, adenylate kinase and nucleoside diphosphate kinase, are also co-expressed on surfaces of endothelial cells, lymphocytes and other cell types and finely control local nucleotide concentrations via backward ATP-regenerating pathway (Fig. 1 B ) (Yegutkin et al. 2002). The generated adenosine, in turn, has a non-redundant counteracting role in the attenuation of inflammation and mediates cardioprotective, vasodilatory, angiogenic and other responses via interaction with own G-protein-coupled receptors (Bours et al. 2006). Extracellular adenosine is then either transported into the cell by nucleoside transporters or further inactivated to inosine via ecto-adenosine deaminase reaction (Yegutkin et al. 2002). Together, the extracellular nucleotide turnover depends on functional interactions between distinct processes including:
- transient release of ATP, ADP and other agonists;
- triggering of signaling events via nucleotide- and nucleoside-selective receptors;
- ectoenzymatic inactivation;
- nucleoside uptake by the cell.

The concept of a purinergic signalling system is now widely appreciated, and studies on pathophysiology and therapeutic potential of extracellular purines represent a novel and rapidly expanding field (Burnstock, 2006). In particular, recent findings provide evidence for important roles of circulating nucleotides in the regulation of platelet reactivity, haemostasis and blood flow under exercising conditions. Regular exercise training is consistently associated with a variety of favourable alterations in cardio-vascular function, including reduced heart rate and increased maximal oxygen uptake, reduced blood pressure, activation of fibrinolysis and lower platelet activation. However, unfavourable haemostatic changes might occur at extreme exercise and environmental conditions that predispose to occlusive thrombus formation in coronary or cerebral vessels, and the extremely rare phenomenon of sudden cardiac death during exertion. Platelet activation and recruitment, followed by haemostatic plug formation, is generally initiated either via formation of thromboxane-A2 by cyclooxygenase or secretion of ADP from dense granules with subsequent activation of platelet ADP-selective P2Y1/P2Y12 receptors. In turn, vascular endothelium controls platelet reactivity and prevents thrombus formation via three pathways, including nitric oxide and prostaglandin-I2 synthesis and ADP scavenging via NTPDase activity (Burnstock, 2006).

Recently, we have shown that intravascular nucleotide turnover is acutely activated both in endurance-trained and sedentary subjects during performance of maximal cycling exercise (Yegutkin et al. 2007). A salient finding of this work is the demonstration that plasma from exercising humans, but not from resting control samples, up-regulates the expression level of the platelet activation marker P-selectin and that these prothrombotic effects can be attenuated after scavenging nucleotides by exogenous apyrase (Fig. 2). Subsequent reverse-phase high-performance liquid chromatographic analysis directly confirmed a significant increase in plasma ADP during exercise. This work additionally pointed to a role of ADP in platelet function beyond its immediate activity as a primary agonist. Probably, other synergistic factors like adrenaline (epinephrine) or some chemokines are released simultaneously with ADP that, in conjunction with the increased blood flow, would provide the stimuli for platelet activation during exercise.
While preferential activation of the coagulation cascade may predispose exercising subjects to the enhanced risk of intravascular thrombosis formation, other accompanying haemostatic changes such as activation of fibrinolysis and increased blood flow should work to counterbalance it. Blood flow and its surrogate oxygen delivery regulation are generally thought to result from the interplay of neural, myogenic and metabolic signals. A number of observations raised the possibility that purinergic signalling can also be implicated in the precise regulation of oxygen supply to contracting muscle under exercising and other hypoxic and hypercapnic conditions. Specifically, in addition to serving as an efficient oxygen carrier, the red blood cells act as sensors and controllers of local blood flow via transient release of ATP in proportion to the degree of haemoglobin deoxygenation (González-Alonso et al. 2002; Ellsworth, 2004). The released ATP subsequently induces a conducted vasodilatatory response upstream and regulates oxygen supply to contracting muscles via binding to the endothelial P2Y1/P2Y2 receptors and stimulation of vascular endothelium to release nitric oxide and arachidonic acid metabolites (Ellsworth, 2004; Burnstock, 2006).
Identification of a network of soluble purine-converting enzymes freely circulating in the bloodstream adds another level of complexity to understanding the regulatory mechanisms of purine homeostasis within the vasculature. We have shown that two soluble nucleotide-inactivating enzymes, nucleotide pyrophosphatase/ phospho-diesterase (NPP) and NTPDase, constitutively circulate in the human bloodstream, and we have further demonstrated that their activities are transiently up-regulated during strenuous exercise by 20–25 and 80–100%, respectively (Yegutkin et al . 2007). The exercise-mediated increase revealed in serum NPP activity may allow the by-passing of the generation of a principal platelet-recruiting agent ADP, via direct conversion of circulating ATP into AMP and PPi. Furthermore, concurrent activation of another soluble nucleotidase NTPDase might represent a novel and currently unappreciated effector system contributing, along with vascular endothelial NTPDases, to the termination of acute prothrombotic effects of ADP under hyperaemic exercising conditions. Of note is the fact that the recombinant soluble form of human NTPDase/CD39 is currently considered a promising aspirin-insensitive antithrombotic drug, which potently inhibits platelet reactivity under various experimental prothrombotic conditions. Therefore, data on constitutive presence of soluble NTPDase in human blood and its up-regulation during exhaustive exercise may open up further research for future therapeutic applications of this major ADP-inactivating nucleotidase as a ‘natural antithrombotic enzyme’ for anti-platelet therapy in hypoxia-associated and other vascular diseases.
In summary, transient exercise-mediated increases in circulating ATP and ADP levels, together with concurrent up-regulation of soluble nucleotide-inactivating activities induced by endurance training, may represent an efficient control system that finely regulates both tissue O2 delivery and platelet reactivity in healthy subjects. On the other hand, acute disturbances in the pattern of intravascular nucleotide turnover occurring during exhaustive exertion might contribute, in conjunction with other prothrombotic synergistic factors, to the enhanced risk of cardiovascular morbidity and mortality, especially in the elderly and sedentary subjects suffering from endothelial dysfunction and insufficient release of anti-platelet compounds.
Bours MJL, Swennen ELR, Di Virgilio F, Cronstein BN & Dagnelie PC (2006). Adenosine 5′-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacology & Therapeutics 112 , 358–404.
Burnstock G (2006). Pathophysiology and therapeutic potential of purinergic signalling. Pharmacol Rev 58 , 58–86.
Ellsworth ML (2004). Red blood cell-derived ATP as a regulator of skeletal muscle perfusion. Med Sci Sports Exerc 36 , 35–41.
González-Alonso J, Olsen DB & Saltin B (2002). Erythrocyte and the regulation of human skeletal muscle blood flow and oxygen delivery: role of circulating ATP. Circ Res 91 , 1046–1055.
Yegutkin GG, Henttinen T, Samburski SS, Spychala J & Jalkanen S (2002). The evidence for two opposite, ATP-generating and ATP-consuming, extracellular pathways on endothelial and lymphoid cells. Biochem J 367 , 121–128.
Yegutkin GG, Samburski SS, Mortensen SP, Jalkanen S & González-Alonso J (2007). Intravascular ADP and soluble nucleotidases contribute to acute prothrombotic state during vigorous exercise in humans. J Physiol 579 , 553–564.
Site search
Content type.
- Latest News
- Upcoming Events
- Team Members
- Honorary Fellows
- Search Menu
- Sign in through your institution
- Advance Articles
- Clinical Case Studies
- Journal Club
- Clinical Chemistry Podcasts
- Clinical Trainee Council
- Special Issues
- Clinical Chemistry Guide to Scientific Writing
- Clinical Chemistry Guide to Manuscript Review
- Author Guidelines
- Submission Site
- Self-Archiving Policy
- Call for Papers
- Why Publish?
- About Clinical Chemistry
- Editorial Board
- Advertising & Corporate Services
- Journals on Oxford Academic
- Books on Oxford Academic
Article Contents
Materials and methods.
- < Previous

Human Plasma ATP Concentration
- Article contents
- Figures & tables
- Supplementary Data
Mark W Gorman, Eric O Feigl, Charles W Buffington, Human Plasma ATP Concentration, Clinical Chemistry , Volume 53, Issue 2, 1 February 2007, Pages 318–325, https://doi.org/10.1373/clinchem.2006.076364
- Permissions Icon Permissions
Background: Human plasma ATP concentration is reported in many studies as roughly 1000 nmol/L. The present study tested the hypothesis that the measured plasma ATP concentration is lower if ATP release from formed blood elements is inhibited during blood sample processing. A second hypothesis was that pretreatment with aspirin to inhibit platelets would reduce the measured plasma concentration of ATP.
Methods: Blood was sampled from the antecubital vein in 20 healthy individuals 30 and 60 min after ingestion of aspirin (325 mg) or placebo. Aliquots of each blood sample were added to the usual EDTA/saline solution to inhibit ATP catabolism, or to a new stabilizing solution designed to both stop ATP catabolism and inhibit ATP release from blood elements. The stabilizing solution contained NaCl, EDTA, tricine buffer, KCl, nitrobenzylthioinosine, forskolin, and isobutylmethylxanthine. Plasma ATP was measured with the luciferin–luciferase assay with standard additions in each sample to determine ATP content. Hemoglobin concentration was used as an index of sample hemolysis, and the plasma ATP concentration was corrected for the hemolysis component.
Results: Aspirin pretreatment had no effect on plasma ATP concentrations. However, use of the stabilizing solution resulted in mean (SD) ATP concentrations 8-fold lower than the use of EDTA alone [28 (16) vs 236 (201) nmol/L; P <0.001].
Conclusion: When precautions are taken to inhibit ATP release from blood elements during sample preparation, human venous plasma ATP concentration is much lower than previously reported.
ATP release can occur during various physiological and pathophysiological events such as ischemia, hypoxia, platelet aggregation, sympathetic nerve stimulation, or cellular damage ( 1 )( 2 )( 3 )( 4 )( 5 ). ATP can affect thromboregulation and stimulate the immune cells responsible for asthma attacks ( 6 )( 7 ). ATP has also been proposed as a mediator of vasodilation during ischemia, hypoxia, and exercise. According to this hypothesis, low oxygen tension provokes ATP release from erythrocytes, as has been demonstrated in vitro ( 8 )( 9 ). ATP (or its metabolite ADP) subsequently binds to endothelial P2Y 1 receptors, resulting in vasodilation ( 10 )( 11 ). Recent studies have provided preliminary support for the hypothesis by demonstrating that venous plasma ATP concentration increases during exercise in human skeletal muscle ( 12 ) and in the canine heart ( 13 ).
A plasma ATP assay ( 14 ) developed for the aforementioned dog study measured mean dog plasma ATP concentrations of 25–50 nmol/L ( 13 ). These concentrations are far lower than reported human venous plasma ATP concentrations, which are generally in the 1 μmol/L range (Table 1 1 ). The purpose of the present study was to determine whether human plasma ATP concentration measured with the revised ATP assay is significantly below the micromolar range. Additional aims were to determine whether pretreatment with aspirin to inhibit platelets lowers measured plasma ATP concentration, and whether a 2nd blood sample, drawn at a later time, yields lower ATP concentrations than the first ( 24 ).
Sample treatment is critical to accurate measurement of plasma ATP concentration. EDTA arrests ATP catabolism and is routinely used in plasma ATP assays ( 27 ). However, EDTA does not prevent potential ATP release from erythrocytes or platelets during sample preparation. Gorman et al. ( 14 ) collected blood samples in a stabilizing solution designed to minimize ATP release by platelets and erythrocytes and prevent plasma ATP catabolism. The stabilizing solution contains EDTA to inhibit ATPases and nitrobenzylthioinosine (NBTI) 1 to inhibit ATP release from erythrocytes ( 8 ). Platelets are stabilized by increasing intracellular cAMP with forskolin and by inhibiting cAMP phosphodiesterase with isobutylmethylxanthine (IBMX) in the stabilizing solution. The current study compared ATP concentrations obtained from samples prepared with this stabilizing solution with concentrations from samples prepared with EDTA alone.
Hemolysis is another variable that may increase measured plasma ATP concentrations ( 14 )( 21 ). Erythrocytes have millimolar cytosolic ATP concentrations, so that even a small amount of hemolysis can significantly increase plasma ATP concentration. Plasma hemoglobin concentration is used to estimate the degree of sample hemolysis ( 28 ). The ATP and hemoglobin concentrations from freshly hemolyzed erythrocyte solutions are used to calculate the sample ATP concentration attributable to hemolysis ( 14 ).
This study was approved by the University of Washington human subjects review committee. Study participants were 20 healthy adult paid volunteers [10 female, 10 male; mean (SD) age, 39.4 (14.1); range, 21–75 years] who had not taken aspirin, ibuprofen, or cold medications within the previous 2 weeks. After participants gave written informed consent, they were randomly given either aspirin (325 mg, orally) or a placebo. For each participant, a 9-mL blood sample was drawn from an antecubital vein after a 30-min sitting period, and then 30 min later a 2nd sample was drawn from the opposite arm. The arm chosen first was assigned randomly. Both the investigator drawing blood samples and the investigator measuring plasma ATP concentration were blinded to the aspirin status of the sample donor.
blood sampling protocol
A rubber tourniquet was placed on the upper arm to assist in venipuncture. The blood collection set (Becton Dickinson 367281) used to draw blood samples consisted of a 21-guage ¾-inch needle attached to 12 inches of plastic tubing. Blood samples (∼9 mL) were manually drawn during ∼20 s into 10-mL plastic syringes (Becton Dickinson 301604) containing 60 μL heparin (60 units) for anticoagulation.
blood sample treatment
Immediately after a blood sample was drawn, sample volume in excess of 8 mL (∼1 mL) was expressed from the syringe into a test tube and subsequently used for hematocrit determination. Using the volume markings on the syringe, we added 4-mL blood aliquots to each of 2 plastic tubes containing 5.4 mL of 2 different diluent solutions at room temperature. One tube, referred to as the stabilizing solution sample, contained the stabilizing solution developed by Gorman et al. ( 14 ), containing, per liter, 118 mmol NaCl, 5 mmol KCl, 40 mmol tricine buffer, 4.15 mmol EDTA, 5 nmol NBTI, 10 μmol forskolin, and 100 μmol IBMX, pH adjusted to 7.4 with 2 mol/L KOH. The other tube, referred to as the EDTA-only sample, contained 4.15 mmol/L EDTA in isotonic saline. Blood was gently ejected from the syringe down the side of the tubes to avoid hemolysis. The blood:diluent solution volume ratio matched the ratio used in prior dog studies ( 13 )( 14 ) and provided sufficient volume for 4 subsequent ATP measurements plus a hemoglobin assay (see below). Tubes were capped and gently inverted twice for mixing. Elapsed time from the beginning of blood sample withdrawal to mixing with stabilizing solution or EDTA was ∼1 min. Blood/diluent tubes were immediately centrifuged (13 000 g ) for 2 min at room temperature, and then 5 mL of supernatant was pipetted from these tubes and immediately recentrifuged for 2 min to pellet any remaining erythrocytes caught by surface tension. The supernatant of the 2nd centrifugation was transferred to a new tube and used for ATP and hemoglobin measurements. ATP measurements were begun immediately. Although stabilizing solution greatly retards ATP degradation, it does not stop it indefinitely ( 14 ).
The firefly luciferin–luciferase assay was used for determination of plasma ATP concentration. The current assay has been described earlier in detail ( 14 ). Supernatant aliquots (300 μL) from the 2nd centrifugation were added to each of 4 test tubes containing 100 μL ATP standard solution. The ATP standard solutions contained 0, 10, 20, or 30 pmol ATP per 100 μL. The ATP standard solutions were prepared in stabilizing solution but with the pH previously adjusted to 8.75, so that after addition of all reagents including luciferase the sample pH was ∼7.9, the optimal pH for the luciferase reaction (Fig. 1 1 ). For EDTA-only samples the ATP standard solutions were prepared in EDTA/saline without prior pH adjustment.
After a test tube was placed in the luminometer (Berthold model LB 9507), 25 μL Mg 2+ solution (177 mmol/L MgCl 2 , 40 mmol/L tricine, pH 7.75) was added via an automatic injector in the luminometer to counteract the decrease of sample Mg 2+ concentration by EDTA in the samples; 2 s later, 100 μL luciferase reagent (ATP Bioluminescence Assay Kit CLS II: Roche Diagnostics) was added via a 2nd luminometer injector. After 3 s for the reaction to reach a steady state, the cumulative light output in relative light units was measured for 10 s. A blank sample containing 300 μL of either stabilizing solution or EDTA/saline without plasma was used to determine luminescence in the absence of ATP, and the appropriate value was subtracted from all samples.
Sample ATP content was calculated using the method of standard additions. The cumulative relative light units in 10 s for each test tube (containing 0, 10, 20, or 30 pmol added ATP) was plotted vs the amount of added ATP (Fig. 2 2 ). A least-squares regression line was fit to the data, and the ATP content of the unenriched (native) sample was equal to the y intercept divided by the slope (or the absolute value of the x intercept). This ATP content was attributed to the 300-μL sample. Final concentrations of added ATP in the 4 assay tubes were 0, 19.0, 38.1, and 57.1 nmol/L.
correction for hemolysis
Sample hemoglobin concentration was used as an index of hemolysis. An aliquot of the same supernatant used for ATP determination was used without dilution for hemoglobin concentration determination ( 29 ). Absorbance (A) was measured at 380, 415, and 450 nm with a Bausch and Lomb Spectronic 70 spectrophotometer. Hemoglobin concentration (milligrams per liter) was calculated as 10[16.72 A 415 − 8.36 A 380 − 8.36 A 450 ] ( 13 )( 28 ). To determine the ATP concentration attributable to hemolysis, heparinized blood samples were collected from 2 individuals (without stabilizing solution) and immediately centrifuged. The plasma layer and buffy coat were removed by aspiration. The erythrocyte pellet was resuspended in isotonic saline and centrifuged again. The saline supernatant was removed by aspiration, and various volumes of the erythrocyte pellet (2–40 μL) were lysed in 5 mL distilled water containing 3 mmol/L EDTA (to stabilize the ATP concentration); 25 μL of this lysate was then diluted in 3 mL stabilizing solution, and the ATP content in 300 μL of this solution was determined using the 4-point standard addition method described above. ATP concentration was calculated by attributing the ATP content to the 300 μL sample volume. Hemoglobin concentration was determined in the same solution. ATP concentration was plotted vs hemoglobin concentration to create a hemolysis correction curve (Fig. 3 3 ).
calculation of plasma atp concentration
Sample ATP content (pmol per 300 μL sample) was multiplied by 3.33 to arrive at the concentration (ATP total , nmol/L). The concentration of ATP in the sample due to hemolysis (ATP hemo , nmol/L) was determined from the sample hemoglobin concentration and the hemolysis correction curve and was subtracted from ATP total . Plasma ATP concentration was arrived at after correcting for dilution. Thus, plasma [ATP] = (ATP total − ATP hemo ) × (1.35 + 1 − HCT)/(1 − HCT), where 1.35 is the ratio of stabilizing solution (or EDTA/saline) volume to blood sample volume, and HCT is the fractional hematocrit.
statistical analysis
Three-way ANOVA using aspirin status, sample time, and diluent solution composition as factors revealed that the only significant differences in sample ATP concentration were between stabilizing solution and EDTA-only samples ( P <0.001). Because the data did not show a gaussian distribution, further paired comparisons (sample time, stabilizing solution vs EDTA) were made with the nonparametric Wilcoxon matched-pairs test. Placebo and aspirin results (unpaired) were compared with the nonparametric Mann–Whitney test. Correlation between stabilizing solution and EDTA-only results in the same samples was determined with the nonparametric Spearman correlation. P <0.05 was considered statistically significant. Statistical tests were done with GraphPad Prism software. Results in the text and tables are presented as mean and SD.
The effects of temperature and pH on the luciferase reaction are presented in Fig. 1 1 ( 14 ). Maximum light output is achieved at room temperature and a pH of 7.75–7.95. The graphical determination of plasma ATP concentration is illustrated in Fig. 2 2 . Results are shown for the same blood sample with both stabilizing solution and EDTA only. The native samples are points on the y axis with zero added ATP. Light output as a function of sample ATP content is highly linear in both cases. Because of sample pH optimization, the slope is higher for the stabilizing solution sample than for the EDTA-only sample.
Results of the placebo vs aspirin groups and stabilizing solution vs EDTA only are presented in Fig. 4 4 and Table 2 2 . The 2 samples drawn 30 min apart were not significantly different in any subgroup (Table 2 2 ) and have therefore been combined in Fig. 4 4 , which illustrates that aspirin pretreatment had no effect on plasma ATP concentration. On the other hand, use of stabilizing solution resulted in ATP concentrations 8-fold lower than EDTA alone ( P <0.001).
Paired EDTA and stabilizing solution results from the same blood samples (n = 40, combined aspirin and placebo groups) are presented in Fig. 5 5 . There was no correlation between the 2 values (Spearman r = −0.23; P = 0.15).
The ATP concentrations resulting from intentional hemolysis of small volumes of human erythrocytes are shown in Fig. 3 3 . This relationship between supernatant hemoglobin concentration and ATP concentration was used to correct sample ATP concentrations for hemolysis. For all stabilizing solution samples (n = 40) the mean (SD) supernatant (plasma + stabilizing solution) hemoglobin concentration was 2.7 (1.6) mg/L. Without hemolysis correction the plasma ATP concentration was 64 ( 23 ) nmol/L. After individual correction for hemolysis the plasma ATP concentration was 28 ( 16 ) nmol/L. Thus, hemolysis correction reduced the ATP concentration in stabilizing solution samples by 56%. In 4 of the 20 study participants high plasma turbidity or coloration prevented an accurate plasma hemoglobin determination. These samples were assigned the mean hemoglobin concentration from all samples from the remaining 16 participants. The supernatant hemoglobin concentration in EDTA-only samples [2.5 (1.5) mg/L] was not different from that of stabilizing solution samples [2.7 (1.6) mg/L]. These values correspond to undiluted plasma hemoglobin concentrations of 8.3 mg/L in EDTA-only samples and 9.0 mg/L in stabilizing solution samples.
The most important conclusion from this study is that addition of the stabilizing solution to human blood samples leads to measured plasma ATP concentrations 8-fold lower than the use of EDTA alone. Use of stabilizing solution and correction for ATP released by hemolysis results in measured plasma ATP concentrations [28 ( ( 16 )) nmol/L] that are much lower than those reported in previous studies (see Table 1 1 ). Because many artifacts can increase plasma ATP, the lower values are more likely to be the correct ones. Other conclusions are that aspirin pretreatment does not influence the measurement of plasma ATP concentration, and a 2nd blood sample drawn 30 min after the first results in measurements that are not significantly different.
An accurate plasma ATP assay should prevent both plasma ATP catabolism and additional ATP release by blood formed elements during sample preparation. EDTA prevents plasma ATP breakdown ( 14 )( 27 ) and is routinely added to blood samples in plasma ATP assays. The stabilizing solution in the current assay includes the nucleoside transport inhibitor NBTI, which also inhibits erythrocyte ATP release ( 8 ). Platelets are stabilized by inclusion of forskolin to increase cAMP concentration and IBMX to inhibit cAMP phosphodiesterase ( 30 )( 31 ). Use of a stabilizing solution is analogous to the approach developed for plasma adenosine assays in which blood is mixed with inhibitors of adenosine formation, uptake, and deamination ( 32 )( 33 )( 34 ).
Although the use of EDTA alone instead of stabilizing solution increased the measured ATP concentration 8-fold on average, the correlation between EDTA-only and stabilizing solution ATP concentrations in the same blood samples was nonexistent (Fig. 5 5 ). This result may be explained by centrifugation of EDTA-only samples releasing a large amount of ATP from platelets or erythrocytes. Assuming that the ATP concentrations with stabilizing solution are correct, Fig. 5 5 demonstrates that using an EDTA-only assay and dividing the resulting concentration by 8 will not provide a reliable estimate of ATP concentration in individual samples.
A potentially important difference between the stabilizing solution and EDTA/saline was that the stabilizing solution was buffered to pH 7.4. The EDTA/saline solution typically had a pH of 4.6. It is unlikely, however, that a lower pH induced the release of ATP in the EDTA/saline samples. In dog blood samples in which pH 7.4 buffered stabilizing solution was compared with and without forskolin/IBMX, forskolin and IBMX decreased measured ATP values at a constant stabilizing solution pH ( 14 ). Hemoglobin concentrations in EDTA-only and stabilizing solution samples in the present study were not different, indicating that pH differences did not induce hemolysis.
Sample buffering clearly has an influence on the light output from the luciferase reaction, with buffered samples having higher output for a given ATP concentration (Figs. 1 1 and 2 2 ). Most of the buffering for optimum luciferase reaction pH (∼7.9) was achieved in stabilizing solution samples by adjusting the pH of the ATP standard solution added to the luminometer tubes. Although sample pH and possibly other components of the stabilizing solution (forskolin, IBMX, etc.) influence the light output of the luciferase reaction, this does not explain the lower ATP concentrations in stabilizing solution samples. Both EDTA-only and stabilizing solution samples exhibited highly linear standard curves when light output was plotted vs ATP content (Fig. 2 2 ). The virtue of the standard addition technique is that all 4 samples in these plots are identical except for ATP content. Any effect of sample composition on light output (other than ATP) is therefore common to all samples and does not influence the assay result. The effect of stabilizing solution on sample ATP concentration occurs during sample processing and is independent of effects on the luciferase reaction.
Another factor contributing to low plasma ATP concentrations in the current study is correction for hemolysis. The high cytosolic ATP concentration in erythrocytes means that hemolysis invisible to the naked eye can significantly increase plasma ATP concentration. Plasma hemoglobin concentration was used as an index of hemolysis. Harkness et al. ( 21 ) plotted plasma ATP concentrations vs plasma hemoglobin concentrations in a group of samples and extrapolated to zero hemoglobin for an estimate of hemolysis-free plasma ATP concentration. The hemolysis correction technique in the current study has the virtue of being applicable to individual samples. In samples treated with stabilizing solution, hemolysis was on average responsible for roughly half of the sample ATP concentration (56%). It is uncertain, however, whether the low plasma hemoglobin concentrations in this study represent fresh hemolysis. The hemoglobin concentration in circulating plasma may be greater than zero; if so, the present method overcorrects for hemolysis, and the true plasma ATP concentration may be somewhat higher. Thus, the true mean plasma ATP concentration in the current study may be between 28 nmol/L (with hemolysis correction) and 64 nmol/L (no hemolysis correction).
The sample hemoglobin concentrations in the current study [overall mean, 2.6 (1.5) mg/L] incorporated dilution of plasma with stabilizing solution or EDTA/saline. When corrected for this dilution, mean hemoglobin concentration in the undiluted plasma was 8.6 mg/L, which represents ∼0.006% hemolysis (assuming 140 g hemoglobin per L blood). Even modest hemolysis can clearly have a large influence on plasma ATP concentration, an effect that may account for some of the high values reported in the literature (Table 1 1 ). It is essential to measure the hemoglobin concentration in every sample. Samples with >∼10 mg/L hemoglobin after dilution with stabilizing solution (∼32.5 mg/L in undiluted plasma) should probably be discarded because of the large hemolysis correction that would be required.
A given plasma hemoglobin concentration in human samples required roughly twice the plasma ATP correction that was necessary in dog plasma ( 14 ). This finding is consistent with the high ATP content in human erythrocytes compared with those of dogs ( 35 ). The slope of the plot in Fig. 3 3 indicates an erythrocyte ATP content of 3.94 μmol ATP per g hemoglobin, similar to human erythrocyte ATP contents detected by other laboratories [3.7 ( 8 ), 4.24( 36 ), and 3.94( 37 )]. Other plasma hemoglobin assays ( 28 ) may be used in place of the Harboe ( 29 ) technique chosen for this study and may prove to be superior in samples with low hemolysis. Each laboratory should use the chosen hemoglobin assay to generate a hemolysis ATP correction curve similar to Fig. 3 3 .
One hypothesis was that the negative effects of aspirin on platelet aggregation might reduce platelet ATP release and lead to lower plasma ATP concentrations, especially in samples treated with EDTA only. The results clearly do not support this hypothesis, because aspirin pretreatment had no effect on plasma ATP concentration in either saline/EDTA or stabilizing solution. Aspirin had no effect in samples taken either 30 or 60 min after aspirin ingestion. Thirty min after oral administration is sufficient for aspirin-induced suppression of platelet thromboxane B 2 production ( 38 ).
Ryan et al. ( 24 ) found that when blood sampling was repeated 15–30 min after obtaining the initial sample, the 2nd sample contained 42% less ATP. The present study found no significant changes in ATP concentration measured in samples collected 30 min apart with either stabilizing solution or EDTA/saline, regardless of whether or not aspirin was present.
The low plasma ATP concentrations in the current study are not the result of low recoveries. Plasma ATP loss stops as soon as blood is treated with EDTA ( 14 )( 27 ). The current assay recovers 96.2% of exogenously added ATP ( 14 ). Some plasma ATP is bound to albumin and is not detected by the luciferin–luciferase assay ( 39 ). In rat plasma, heat denaturation increased average plasma ATP concentration from 93 to 150 nmol/L ( 39 ). Similar albumin binding probably occurs in human plasma, but has not been measured to date. The present results do not include albumin-bound ATP. The unbound ATP concentration is probably indicative of the ATP that acts on physiological purinergic receptors.
The use of syringe volume markings to measure 4-mL samples of blood in the current study is less accurate than the use of pipettes. However, pipetting these blood samples would require additional time and manipulation of blood that had not yet been treated with either EDTA or stabilizing solution, resulting in additional ATP catabolism and possibly additional hemolysis or platelet activation. A modest amount of volume accuracy was purposely sacrificed to avoid these complications. Volume inaccuracies should be randomly distributed among EDTA-only and stabilizing solution samples.
All blood samples in this study were drawn from the antecubital veins. Because oxygen tension influences ATP release from erythrocytes ( 8 )( 9 ), samples drawn from other locations with different venous oxygen tensions may result in different ATP concentrations. Local ATP concentrations, particularly near aggregating platelets or nerve endings, may be considerably higher than the circulating venous concentrations reported here.
In summary, addition of blood samples to a stabilizing solution designed to stabilize plasma ATP concentration resulted in human plasma ATP concentrations 8-fold lower than treatment with EDTA alone. Aspirin pretreatment did not affect plasma ATP concentration. ATP concentration did not change in blood samples drawn 30 min apart. Correction for hemolysis-induced ATP release decreased plasma ATP concentration by 56%. These results indicate that the true resting human venous plasma ATP concentration is far lower than previously reported and is ∼28 nmol/L.
Human plasma ATP studies. 1
A compilation of studies reporting normal human plasma ATP concentrations. All concentrations have been converted to nmol/L. The Notes column indicates additives to the blood sample, the type of assay (luciferase or HPLC), and other details of sample preparation or results. Samples are venous unless indicated otherwise. Some concentrations (∼) were estimated from figures.
EHNA, erythro-6-amino-9-(2-hydroxy-3-nonyl)-purine hydrochloride, βTG, β-thromboglobulin.
( A ), effect of sample temperature on bioluminescence at a constant ATP concentration (10 nmol/L).
Maximum light output and minimum temperature dependence are achieved near room temperature. ( B ), effect of sample pH on bioluminescence. We added 300 μL of stabilizing solution at various pH values to 100 μL of 100 nmol/L ATP in distilled water; 25 μL of 177 mmol/L MgCl 2 and 100 μL of luciferase reagent were added by automatic injection in the luminometer. The pH and light output of the resulting mixtures are plotted. Maximum light output with minimum pH dependence was obtained in the pH range 7.75–7.95. Reproduced with permission from reference 14, copyright John Wiley & Sons Ltd., 2003.
The standard addition technique for determination of ATP concentration in unknown plasma samples.
Aliquots (4 mL) of the same blood sample were analyzed after treatment with 5.4 mL stabilizing solution or saline/EDTA. From the resulting diluted plasma samples, 4 assay tubes were prepared containing 0, 10, 20, and 30 pmol added ATP. Luminescence from the luciferin–luciferase reaction (after subtracting blank values) was plotted vs added ATP. The x intercept of the linear regression line indicates the ATP content of the native plasma tube (zero added ATP). The slope is lower in EDTA-only samples because pH was not optimized for the luciferase reaction. The accuracy of the assay was not affected, because luminescence remains a highly linear function of ATP content. After correction for hematocrit, dilution, and hemolysis, the original plasma concentrations in this sample were 22.4 nmol/L with stabilizing solution and 231 nmol/L with EDTA only.
The influence of hemolysis on sample ATP concentration.
Small volumes (2–40 μL) of erythrocytes from 2 individuals ( open and closed circles ) were freshly lysed in distilled water/EDTA and further diluted in stabilizing solution. ATP concentrations were measured with the standard addition technique, and free hemoglobin concentration was measured spectrophotometrically. The results were fit to a linear regression line constrained through the origin. The results demonstrate that even very low levels of hemolysis significantly increase plasma ATP concentration.
The effects of stabilizing solution and aspirin pretreatment on human plasma ATP concentration.
There were no significant differences between 30-min and 60-min samples, which have been consolidated in this figure. Aspirin pretreatment had no effect on ATP concentration with either stabilizing solution or EDTA only. However, stabilizing solution resulted in plasma ATP concentrations far lower than the use of EDTA alone ( P <0.001 for both placebo and aspirin groups). Numbers within the bars are the number of samples. Error bars indicate SE.
Plasma ATP concentrations.
Values are mean (SD). n = 10 except for all samples, where n = 40. There were no significant differences between aspirin and placebo groups or between the first sample taken 30 min after placebo or aspirin and the second sample taken 30 min after the first sample.
P <0.001 vs EDTA only.
Aliquots of every blood sample (n = 40) were added to both stabilizing solution and EDTA/saline.
The paired plasma ATP concentration results (corrected for hemolysis) are plotted. There is no correlation between the results for the 2 different diluents (Spearman r = −0.23; P value not significant). These data demonstrate that a measurement made in EDTA/saline cannot simply be divided by the ratio of the mean values of the 2 methods (8-fold).
Nonstandard abbreviations: NBTI, nitrobenzylthioinosine; IBMX, isobutylmethylxanthine.
Mills DC, Robb IA, Roberts GC. The release of nucleotides, 5-hydroxytryptamine and enzymes from human blood platelets during aggregation. J Physiol 1968 ; 195 : 715 -729.
Paddle BM, Burnstock G. Release of ATP from perfused heart during coronary vasodilatation. Blood Vessels 1974 ; 11 : 110 -119.
Fredholm BB, Hedqvist P, Lindstrom K, Wennmalm M. Release of nucleosides and nucleotides from the rabbit heart by sympathetic nerve stimulation. Acta Physiol Scand 1982 ; 116 : 285 -295.
Gordon JL. Extracellular ATP: effects, sources and fate. Biochem J 1986 ; 233 : 309 -319.
Borst MM, Schrader J. Adenine nucleotide release from isolated perfused guinea pig hearts and extracellular formation of adenosine. Circ Res 1991 ; 68 : 797 -806.
Pelleg A, Schulman ES. Adenosine 5′-triphosphate axis in obstructive airway diseases [Review]. Am J Ther 2002 ; 9 : 454 -464.
Vial C, Pitt SJ, Roberts J, Rolf MG, Mahaut-Smith MP, Evans RJ. Lack of evidence for functional ADP-activated human P2X1 receptors supports a role for ATP during hemostasis and thrombosis. Blood 2003 ; 102 : 3646 -3651.
Bergfeld GR, Forrester T. Release of ATP from human erythrocytes in response to a brief period of hypoxia and hypercapnia. Cardiovasc Res 1992 ; 26 : 40 -47.
Ellsworth ML, Forrester T, Ellis CG, Dietrich HH. The erythrocyte as a regulator of vascular tone. Am J Physiol 1995 ; 269 : H2155 -H2161.
Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev 1998 ; 50 : 413 -492.
Gorman MW, Ogimoto K, Savage MV, Jacobson KA, Feigl EO. Nucleotide coronary vasodilation in guinea pig hearts. Am J Physiol Heart Circ Physiol 2003 ; 285 : H1040 -H1047.
Gonzalez-Alonso J, Olsen DB, Saltin B. Erythrocyte and the regulation of human skeletal muscle blood flow and oxygen delivery: role of circulating ATP. Circ Res 2002 ; 91 : 1046 -1055.
Farias M, III, Gorman MW, Savage MV, Feigl EO. Plasma ATP during exercise: possible role in regulation of coronary blood flow. Am J Physiol Heart Circ Physiol 2005 ; 288 : H1586 -H1590.
Gorman MW, Marble DR, Ogimoto K, Feigl EO. Measurement of adenine nucleotides in plasma. Luminescence 2003 ; 18 : 173 -181.
Forrester T, Lind AR. Identification of adenosine triphosphate in human plasma and the concentration in the venous effluent of forearm muscles before, during and after sustained contractions. J Physiol 1969 ; 204 : 347 -364.
Forrester T. The identification of adenosine triphosphate in fresh human plasma. J Physiol 1969 ; 200 : 53P -54P.
Parkinson PI. The effect of graduated exercise on the concentration of adenine nucleotides in plasma. J Physiol 1973 ; 234 : 72P -74P.
Jabs CM, Ferrell WJ, Robb HJ. Microdetermination of plasma ATP and creatine phosphate concentrations with a luminescence biometer. Clin Chem 1977 ; 23 : 2254 -2257.
Moss AH, Solomons CC, Alfrey AC. Elevated plasma adenine nucleotide levels in chronic renal failure and their possible significance. Proc Clin Dial Transplant Forum 1979 ; 9 : 184 -188.
Harkness RA, Simmonds RJ, Coade SB. Purine transport and metabolism in man: the effect of exercise on concentrations of purine bases, nucleosides and nucleotides in plasma, urine, leucocytes and erythrocytes. Clin Sci (Lond) 1983 ; 64 : 333 -340.
Harkness RA, Coade SB, Webster AD. ATP, ADP and AMP in plasma from peripheral venous blood. Clin Chim Acta 1984 ; 143 : 91 -98.
Born GV, Kratzer MA. Source and concentration of extracellular adenosine triphosphate during haemostasis in rats, rabbits and man. J Physiol 1984 ; 354 : 419 -429.
Capecchi PL, Laghi PF, Sodi N, Chiavetta M, Sensi S, De Lalla A, et al. Increase in plasma levels of adenosine and adenine nucleotides after intravenous infusion of buflomedil in humans. J Cardiovasc Pharmacol 1995 ; 25 : 35 -39.
Ryan LM, Rachow JW, McCarty BA, McCarty DJ. Adenosine triphosphate levels in human plasma. J Rheumatol 1996 ; 23 : 214 -219.
Lader AS, Prat AG, Jackson GR, Jr, Chervinsky KL, Lapey A, Kinane TB, et al. Increased circulating levels of plasma ATP in cystic fibrosis patients. Clin Physiol 2000 ; 20 : 348 -353.
Rosenmeier JB, Hansen J, Gonzalez-Alonso J. Circulating ATP-induced vasodilatation overrides sympathetic vasoconstrictor activity in human skeletal muscle. J Physiol 2004 ; 558 : 351 -365.
Holmsen H, Holmsen I, Bernhardsen A. Microdetermination of adenosine diphosphate and adenosine triphosphate in plasma with firefly luciferase system. Anal Biochem 1966 ; 17 : 456 -473.
Malinauskas RA. Plasma hemoglobin measurement techniques for the in vitro evaluation of blood damage caused by medical devices. Artif Organs 1997 ; 21 : 1255 -1267.
Harboe M. A method for determination of hemoglobin in plasma by near-ultraviolet spectrophotometry. Scand J Clin Lab Invest 1959 ; 11 : 66 -70.
Feinstein MB, Fraser C. Human platelet secretion and aggregation induced by calcium ionophores: inhibition by PGE1 and dibutyryl cyclic AMP. J Gen Physiol 1975 ; 66 : 561 -581.
Salzman EW, Rubino EB, Sims RV. Cyclic 3′,5′-adenosine monophosphate in human blood platelets: 3: the role of cyclic AMP in platelet aggregation. Ser Haematol 1970 ; 3 : 100 -113.
Manfredi JP, Sparks HV, Jr. Adenosine’s role in coronary vasodilation induced by atrial pacing and norepinephrine. Am J Physiol Heart Circ Physiol 1982 ; 243 : H536 -H545.
Shryock JC, Boykin MT, Hill JA, Belardinelli L. A new method of sampling blood for measurement of plasma adenosine. Am J Physiol Heart Circ Physiol 1990 ; 258 : H1232 -H1239.
Herrmann SC, Feigl EO. Subtraction method for the high-performance chromatographic measurement of plasma adenosine. J Chromatogr 1992 ; 574 : 247 -253.
Miseta A, Bogner P, Berenyi E, Kellermayer M, Galambos C, Wheatley DN, et al. Relationship between cellular ATP, potassium, sodium and magnesium concentrations in mammalian and avian erythrocytes. Biochim Biophys Acta 1993 ; 1175 : 133 -139.
Olsson T, Gulliksson H, Palmeborn M, Bergstrom K, Thore A. Methodological aspects on the firefly luciferase assay of adenine nucleotides in whole blood and red blood cells. Scand J Clin Lab Invest 1983 ; 43 : 657 -664.
Ecker T, Hitzler WE. Effect of 6-hour exposure to 20 degrees C on the ATP content and other biochemical measures of CPDA-1 packed red cells. Clin Lab 2000 ; 46 : 291 -293.
Patrono C, Ciabattoni G, Patrignani P, Pugliese F, Filabozzi P, Catella F, et al. Clinical pharmacology of platelet cyclooxygenase inhibition. Circulation 1985 ; 72 : 1177 -1184.
Douillet CD, Suy S, Zarzaur BL, Robinson WP, III, Milano PM, Boucher RC, et al. Measurement of free and bound fractions of extracellular ATP in biological solutions using bioluminescence. Luminescence 2005 ; 20 : 435 -441.
Email alerts
Citing articles via.
- Recommend to Your Librarian
- Advertising and Corporate Services
- Journals Career Network
Affiliations
- Online ISSN 1530-8561
- Print ISSN 0009-9147
- Copyright © 2024 Association for Diagnostics & Laboratory Medicine
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
Advertisement intended for health care professionals
The Inflammation Signaling Molecule ATP Regulates Human CD4 + T Cell Functions
- Split-Screen
- Request Permissions
- Cite Icon Cite
- Search Site
Sara Trabanelli , Darina Očadlíková , Sara Gulinelli , Antonio Curti , Francesco di Virgilio , Davide Ferrari , Roberto M. Lemoli; The Inflammation Signaling Molecule ATP Regulates Human CD4 + T Cell Functions. Blood 2010; 116 (21): 3901. doi: https://doi.org/10.1182/blood.V116.21.3901.3901
Download citation file:
- Ris (Zotero)
- Reference Manager
Abstract 3901
Adenosine 5'-triphosphate (ATP) is emerging as an extracellular signaling molecule playing a pivotal role in several cellular processes, through specific cell membrane purinergic P2 receptors (P2Rs). Under physiological conditions, ATP is present in the extracellular space at low concentrations (1-10 nM), whereas during inflammation and tumor cell growth ATP is present in the extracellular space at high concentrations, when 5–10 mM of ATP are quickly released from cytoplasm following plasma membrane damage or membrane stretching. For these reasons, extracellular ATP, via activation of P2Rs, might be an important regulator of inflammatory and immune response.
CD4 + T cells are often exposed to different ATP concentrations in healthy or in injured/inflamed tissues. In the present study, we investigated the expression of purinergic P2 receptors (P2Rs) on human activated and regulatory CD4 + T cells and tested the lymphocyte functions in presence of low (1-10 nM), intermediate (250 nM) and high (1 mM) concentration of extracellular ATP. We assessed CD4 + T cells proliferation, apoptosis, phenotype, cytokine release, migration and matrix/cells adhesion.
We show that activated CD4 + T cells express all P2Rs subtypes, whereas T regs do not express P2X 6 and P2Y 2 . At a functional level, low concentrations of extracellular ATP do not modulate CD4 + T cell functions. An increase in ATP concentration (250 nM) stimulates CD4 + T cells during activation: activated CD4 + T cells enhance their proliferation, the secretion of several cytokines critical for T cell functions (IL-2, IL-1b, IFN-g, IL-8), the expression of adhesion molecules (CD49d and CD54) and the capacity to adhere to cellular matrix or to other cells. T regs seem to be unaffected by 250 nM of ATP. In contrast, high concentrations of ATP (1 mM) “turn off” activated CD4 + T cells and “turn on” T regs . 1 mM of ATP inhibits activation of CD4 + T cells, by enhancing apoptosis and diminishing proliferation, cell-adhesion and the release of pro-inflammatory cytokines. Conversely, 1 mM of ATP attracts T regs and stimulates their proliferation and their capacity to adhere to other cells. Moreover, T regs cultured in presence of 1 mM of extracellular ATP are more efficient in inhibiting T cell proliferation.
In summary, the present data show that the concentration of extracellular ATP regulates CD4 + T cell functions. Low ATP concentrations, as in physiological conditions, do not affect CD4 + T cell functions, whereas any enhancement of ATP concentration alters CD4 + T cell behavior. Specifically, a small increase stimulates CD4 + T cell activation, whereas a high increase inhibits CD4 + T cell activation and promotes the immunosuppression T regs -mediated.
We propose that the present in vitro data might explain how in vivo ATP regulates the behavior of activated CD4 + T cells and T regs in case of inflammation or tumor cell growth. A small enhancement of ATP concentration occurs at the beginning of an inflammatory state or at the first stages of tumor growth; these ATP concentrations alert CD4 + T cells to the presence of a possible damage, which does not yet require T regs involvement. In contrast, in case of severe inflammation, high ATP concentrations might prevent a further involvement of activated CD4 + T cells and promotes T regs recruitment, avoiding hyper-inflammation. In case of advanced stages of tumorigenesis, high ATP concentration might be a tumor-escape mechanism, by killing activated CD4 + T cells and by attracting T regs to surround the tumor.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.
This feature is available to Subscribers Only
- Previous Article
- Next Article
Email alerts
Affiliations.
- Current Issue
- First edition
- Collections
- Submit to Blood
- About Blood
- Subscriptions
- Public Access
- Permissions
- Blood Classifieds
- Advertising in Blood
- Terms and Conditions
American Society of Hematology
- 2021 L Street NW, Suite 900
- Washington, DC 20036
- TEL +1 202-776-0544
- FAX +1 202-776-0545
ASH Publications
- Blood Advances
- Blood Neoplasia
- Blood Vessels, Thrombosis & Hemostasis
- Hematology, ASH Education Program
- ASH Clinical News
- The Hematologist
- Publications
- Privacy Policy
- Cookie Policy
- Terms of Use
This Feature Is Available To Subscribers Only
Sign In or Create an Account
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Yale J Biol Med
- v.55(1); Jan-Feb 1982

Does ATP cross the cell plasma membrane.
Although there is an abundance of evidence which indicates that ATP is released as well as taken up by cells, the concept that ATP cannot cross the cell membrane has tended to prevail. This article reviews the evidence for the release as well as uptake of ATP by cells. The evidence presented by various investigators clearly indicates that ATP can cross the cell membrane and suggests that the release and uptake of ATP are physiological processes.
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.2M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References .
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Long C. The in vitro oxidation of pyruvic and alpha-ketobutyric acids by ground preparations of pigeon brain. The effect of inorganic phosphate and adenine nucleotide. Biochem J. 1943 Jul; 37 (2):215–225. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- HAJDU S, SZENT-GYORGYI A. Action of DOC and serum on the frog heart. Am J Physiol. 1952 Jan; 168 (1):159–170. [ PubMed ] [ Google Scholar ]
- HOLTON FA, HOLTON P. The possibility that ATP is a transmitter at sensory nerve endings. J Physiol. 1953 Mar; 119 (4):50P–51P. [ PubMed ] [ Google Scholar ]
- Glynn IM. Membrane adenosine triphosphatase and cation transport. Br Med Bull. 1968 May; 24 (2):165–169. [ PubMed ] [ Google Scholar ]
- Dieterle Y, Ody C, Ehrensberger A, Stalder H, Junod AF. Metabolism and uptake of adenosine triphosphate and adenosine by porcine aortic and pulmonary endothelial cells and fibroblasts in culture. Circ Res. 1978 Jun; 42 (6):869–876. [ PubMed ] [ Google Scholar ]
- Pull I, McIlwain H. Metabolism of ( 14 C)adenine and derivatives by cerebral tissues, superfused and electrically stimulated. Biochem J. 1972 Feb; 126 (4):965–973. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Silinsky EM, Hubbard JI. Thermal synthesis of amino acids from a simulated primitive atmosphere. Nature. 1973 Jun 15; 243 (5407):404–405. [ PubMed ] [ Google Scholar ]
- Kuroda Y, McIlwain H. Uptake and relase of (14C)adenine derivatives at beds of mammalian cortical synaptosomes in a superfusion system. J Neurochem. 1974 May; 22 (5):691–699. [ PubMed ] [ Google Scholar ]
- White TD. Release of ATP from a synaptosomal preparation by elevated extracellular K+ and by veratridine. J Neurochem. 1978 Feb; 30 (2):329–336. [ PubMed ] [ Google Scholar ]
- Fredholm BB, Vernet L. Release of 3H-nucleosides from 3H-adenine labelled hypothalamic synaptosomes. Acta Physiol Scand. 1979 Jun; 106 (2):97–107. [ PubMed ] [ Google Scholar ]
- Fredholm BB, Hedqvist P. Modulation of neurotransmission by purine nucleotides and nucleosides. Biochem Pharmacol. 1980 Jun 15; 29 (12):1635–1643. [ PubMed ] [ Google Scholar ]
- Kuroda Y. Physiological roles of adenosine derivatives which are released during neurotransmission in mammalian brain. J Physiol (Paris) 1978; 74 (5):463–470. [ PubMed ] [ Google Scholar ]
- Israël M, Lesbats B, Meunier FM, Stinnakre J. Postsynaptic release of adenosine triphosphate induced by single impulse transmitter action. Proc R Soc Lond B Biol Sci. 1976 Jun 30; 193 (1113):461–468. [ PubMed ] [ Google Scholar ]
- Boyd IA, Forrester T. The release of adenosine triphosphate from frog skeletal muscle in vitro. J Physiol. 1968 Nov; 199 (1):115–135. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Forrester T. An estimate of adenosine triphosphate release into the venous effluent from exercising human forearm muscle. J Physiol. 1972 Aug; 224 (3):611–628. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Parkinson PI. Proceedings: The effect of graduated exercise on the concentration of adenine nucleotides in plasma. J Physiol. 1973 Oct; 234 (2):72P–74P. [ PubMed ] [ Google Scholar ]
- Paddle BM, Burnstock G. Release of ATP from perfused heart during coronary vasodilatation. Blood Vessels. 1974; 11 (3):110–119. [ PubMed ] [ Google Scholar ]
- Forrester T, Williams CA. Release of adenosine triphosphate from isolated adult heart cells in response to hypoxia. J Physiol. 1977 Jun; 268 (2):371–390. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Clemens MG, Forrester T. Appearance of adenosine triphosphate in the coronary sinus effluent from isolated working rat heart in response to hypoxia. J Physiol. 1981 Mar; 312 :143–158. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Pearson JD, Gordon JL. Vascular endothelial and smooth muscle cells in culture selectively release adenine nucleotides. Nature. 1979 Oct 4; 281 (5730):384–386. [ PubMed ] [ Google Scholar ]
- Wu PH, Phillis JW. Distribution and release of adenosine triphosphate in rat brain. Neurochem Res. 1978 Oct; 3 (5):563–571. [ PubMed ] [ Google Scholar ]
- Forrester T. Extracellular nucleotides in exercise: possible effect on brain metabolism. J Physiol (Paris) 1978; 74 (5):477–483. [ PubMed ] [ Google Scholar ]
- Pearson JD, Carleton JS, Gordon JL. Metabolism of adenine nucleotides by ectoenzymes of vascular endothelial and smooth-muscle cells in culture. Biochem J. 1980 Aug 15; 190 (2):421–429. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Trams EG. Evidence for ATP action on the cell surface. Nature. 1974 Dec 6; 252 (5483):480–482. [ PubMed ] [ Google Scholar ]
- Rozengurt E, Heppel LA. Reciprocal control of membrane permeability of transformed cultures of mouse cell lines by external and internal ATP. J Biol Chem. 1979 Feb 10; 254 (3):708–714. [ PubMed ] [ Google Scholar ]
- Makan NR, Heppel LA. Control of glycolysis and the pentose phosphate shunt in transformed 3T3 cultures rendered permeable by ATP. J Cell Physiol. 1978 Jul; 96 (1):87–94. [ PubMed ] [ Google Scholar ]
- Dicker P, Heppel LA, Rozengurt E. Control of membrane permeability by external and internal ATP in 3T6 cells grown in serum-free medium. Proc Natl Acad Sci U S A. 1980 Apr; 77 (4):2103–2107. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Wilkinson JH, Robinson JM. Effect of energy-rich compounds on release of intracellular enzymes from human leukocytes and rat lymphocytes. Clin Chem. 1974 Oct; 20 (10):1331–1336. [ PubMed ] [ Google Scholar ]
- Fenn WO, Cobb DM. THE POTASSIUM EQUILIBRIUM IN MUSCLE. J Gen Physiol. 1934 May 20; 17 (5):629–656. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Boyle PJ, Conway EJ. Potassium accumulation in muscle and associated changes. J Physiol. 1941 Aug 11; 100 (1):1–63. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Chaudry IH, Gould MK. Evidence for the uptake of ATP by rat soleus muscle in vitro. Biochim Biophys Acta. 1970; 196 (2):320–326. [ PubMed ] [ Google Scholar ]
- Chaudry IH, Gould MK. Kinetics of glucose uptake in isolated soleus muscle. Biochim Biophys Acta. 1969 May 6; 177 (3):527–536. [ PubMed ] [ Google Scholar ]
- Chaudry IH, Sayeed MM, Baue AE. Uptake of ATP by liver and kidney in vitro. Can J Physiol Pharmacol. 1976 Oct; 54 (5):742–749. [ PubMed ] [ Google Scholar ]
- Chaudry IH, Baue AE. Further evidence for ATP uptake by rat tissues. Biochim Biophys Acta. 1980 Mar 20; 628 (3):336–342. [ PubMed ] [ Google Scholar ]
- Ziegelhöffer A, Fedelesová M, Kostolanský S. Specific ATP action on metabolism of isolated heart. Influence of pH, divalent cation concentration and stability of complexes. Acta Biol Med Ger. 1972; 28 (6):893–900. [ PubMed ] [ Google Scholar ]
- Maxild J. Effect of externally added ATP and related compounds on active transport of p-aminohippurate and metabolism in cortical slices of the rabbit kidney. Arch Int Physiol Biochim. 1978 Aug; 86 (3):509–530. [ PubMed ] [ Google Scholar ]
- Pant HC, Terakawa S, Yoshioka T, Tasaki I, Gainer H. Evidence for the utilization of extracellular [gamma-32P]ATP for the phosphorylation of intracellular proteins in the squid giant axon. Biochim Biophys Acta. 1979 Jan 4; 582 (1):107–114. [ PubMed ] [ Google Scholar ]
- Ayad SR, Hughes RJ. ATP enhances cyclic AMP accumulation by intact P388 mouse lymphoma cells. Biochim Biophys Acta. 1980 Jun 19; 630 (2):193–201. [ PubMed ] [ Google Scholar ]

What Happens to Donated Blood
- Share via Email
- Share on Facebook
- Share on Twitter
- Share on LinkedIn
Your Donated Blood's Journey
Have you ever wondered exactly what happens to the blood you donate at the American Red Cross? Your blood goes on an amazing journey!
You can learn about all the steps your blood goes through before it reaches a recipient in this informative video.
Learn About Each Step of the Blood Journey

- You arrive for your blood donation appointment.
- Health history and mini physical are completed.
- For a whole blood donation, about 1 pint of blood is collected; several small test tubes of blood are also collected for testing.
- Your donation, test tubes and your donor record are labeled with an identical bar code label.
- Your donation is kept on ice before being taken to a Red Cross center for processing; the test tubes go to the lab.

- At our processing center, information about your donation is scanned into a computer database.
- Most whole blood donations are spun in centrifuges to separate it into transfusable components: red cells, platelets, and plasma.
- Plasma may be processed into components such as cryoprecipitate, which helps control the risk of bleeding by helping blood to clot.
- Red cells and platelets are leuko-reduced, which means your white cells are removed in order to reduce the possibility of the recipient having a reaction to the transfusion.
- Each component is packaged as a “unit,” a standardized amount that doctors will use when transfusing a patient.

- In parallel with Step 2, your test tubes arrive at a testing laboratory.
- A dozen tests are performed, to establish the blood type and test for infectious diseases. Learn More About Tests Performed .
- Test results are transferred electronically to the processing center within 24 hours.
- If a test result is positive, your donation will be discarded and you will be notified (our test results are confidential and are only shared with the donor, except as may be required by law).

- When test results are received, units suitable for transfusion are labeled and stored.
- Red cells are stored in refrigerators at 6ºC for up to 42 days.
- Platelets are stored at room temperature in agitators for up to five days.
- Plasma and cryo are frozen and stored in freezers for up to one year.

- Blood is available to be shipped to hospitals 24 hours a day, 7 days a week.
- Hospitals typically keep some blood units on their shelves, but may call for more at any time, such as in case of large scale emergencies.

- An ill or injured patient arrives at a hospital or treatment center.
- Physicians determine whether the patient requires a transfusion and, if so, which type.
- Blood transfusions are given to patients in a wide range of circumstances, including serious injuries (such as in a car crash) surgeries, child birth, anemia, blood disorders, cancer treatments, and many others. See How Blood Donations Help .
- A patient suffering from an iron deficiency or anemia may receive red blood cells to increase their hemoglobin and iron levels, improving the amount of oxygen in the body.
- Patients who are unable to make enough platelets, due to illness or chemotherapy, may receive platelet transfusions to stay healthy.
- Plasma transfusions are used for patients with liver failure, severe infections, and serious burns.
Start Your Own Blood Donation Journey
Types of Blood Donations
How Blood Donations Help
Get the Blood Donor App
Human plasma ATP concentration
Affiliation.
- 1 Department of Physiology and Biophysics, University of Washington, Seattle, WA 98195-7290, USA. [email protected]
- PMID: 17185366
- DOI: 10.1373/clinchem.2006.076364
Background: Human plasma ATP concentration is reported in many studies as roughly 1000 nmol/L. The present study tested the hypothesis that the measured plasma ATP concentration is lower if ATP release from formed blood elements is inhibited during blood sample processing. A second hypothesis was that pretreatment with aspirin to inhibit platelets would reduce the measured plasma concentration of ATP.
Methods: Blood was sampled from the antecubital vein in 20 healthy individuals 30 and 60 min after ingestion of aspirin (325 mg) or placebo. Aliquots of each blood sample were added to the usual EDTA/saline solution to inhibit ATP catabolism, or to a new stabilizing solution designed to both stop ATP catabolism and inhibit ATP release from blood elements. The stabilizing solution contained NaCl, EDTA, tricine buffer, KCl, nitrobenzylthioinosine, forskolin, and isobutylmethylxanthine. Plasma ATP was measured with the luciferin-luciferase assay with standard additions in each sample to determine ATP content. Hemoglobin concentration was used as an index of sample hemolysis, and the plasma ATP concentration was corrected for the hemolysis component.
Results: Aspirin pretreatment had no effect on plasma ATP concentrations. However, use of the stabilizing solution resulted in mean (SD) ATP concentrations 8-fold lower than the use of EDTA alone [28 (16) vs 236 (201) nmol/L; P <0.001].
Conclusion: When precautions are taken to inhibit ATP release from blood elements during sample preparation, human venous plasma ATP concentration is much lower than previously reported.
Publication types
- Comparative Study
- 1-Methyl-3-isobutylxanthine
- Adenosine Triphosphate / blood*
- Blood Chemical Analysis / methods*
- Blood Specimen Collection / methods*
- Edetic Acid
- Hemoglobins / analysis
- Indicators and Reagents
- Middle Aged
- Reference Values
- Hemoglobins
- Adenosine Triphosphate

IMAGES
VIDEO
COMMENTS
The human body uses molecules held in the fats, proteins, and carbohydrates we eat or drink as sources of energy to make ATP. This happens through a process called hydrolysis . After food is digested, it's synthesized into glucose, which is a form of sugar. Glucose is the main source of fuel that our cells' mitochondria use to convert caloric ...
1. Introduction. Classically, the nucleotide adenosine 5′-triphosphate (ATP) has been considered the major energy source in the cell. Through the metabolic breakdown of ingested nutrients, subsequent production of the electron carriers NADH and FADH 2 in the citric acid cycle and the transfer of electrons through the electron transport chain, the mitochondrial ATP synthase synthesize ATP in ...
The nucleotide adenosine 5'-triphosphate (ATP) has classically been considered the cell's primary energy currency. Importantly, a novel role for ATP as an extracellular autocrine and/or paracrine signalling molecule has evolved over the past century and extensive work has been conducted to characterize the ATP-sensitive purinergic receptors expressed on almost all cell types in the body.
Energy released in these reactions is captured as a proton gradient, which is then used to make ATP in a process called chemiosmosis. Together, the electron transport chain and chemiosmosis make up oxidative phosphorylation. The key steps of this process, shown in simplified form in the diagram above, include:
The body is a complex organism, and as such, it takes energy to maintain proper functioning. Adenosine triphosphate (ATP) is the source of energy for use and storage at the cellular level. The structure of ATP is a nucleoside triphosphate, consisting of a nitrogenous base (adenine), a ribose sugar, and three serially bonded phosphate groups. ATP is commonly referred to as the "energy currency ...
ATP is a major player as a signaling molecule in blood microcirculation. It is released by red blood cells (RBCs) when they are subjected to shear stresses large enough to induce a sufficient shape deformation. This prominent feature of chemical response to shear stress and RBC deformation constitutes an important link between vessel geometry ...
To move substances against a concentration or electrochemical gradient, the cell must use energy. This energy is harvested from ATP generated through the cell's metabolism. Active transport mechanisms, collectively called pumps, work against electrochemical gradients. Small substances constantly pass through plasma membranes.
This page titled 5.4: Active Transport is shared under a CC BY license and was authored, remixed, and/or curated by OpenStax. Active transport mechanisms require the use of the cell's energy, usually in the form of adenosine triphosphate (ATP). If a substance must move into the cell against its concentration gradient&….
ATP Definition. Quite simply, adenosine triphosphate (ATP) is the energetic currency of a cell, and it is required for the cell to perform work of any kind, ranging from the synthesis of DNA to sending chemical signals and nerve impulses to the brain. Through metabolic pathways in the body, primarily cellular respiration, ATP is consumed and ...
Specifically, in addition to serving as an efficient oxygen carrier, the red blood cells act as sensors and controllers of local blood flow via transient release of ATP in proportion to the degree of haemoglobin deoxygenation (González-Alonso et al. 2002; Ellsworth, 2004). The released ATP subsequently induces a conducted vasodilatatory ...
In addition, when blood flow to a resting tissue is occluded and then muscle contractions commence, plasma [ATP] does not increase (Fig. 2D). Taken together, our findings suggest that the source of increased plasma [ATP] during exercise is dependent on perfusion and, thus, from cells within or in contact with the blood.
Thus, the true mean plasma ATP concentration in the current study may be between 28 nmol/L (with hemolysis correction) and 64 nmol/L (no hemolysis correction). The sample hemoglobin concentrations in the current study [overall mean, 2.6 (1.5) mg/L] incorporated dilution of plasma with stabilizing solution or EDTA/saline.
Objective— Effects on platelet aggregation of adenosine triphosphate (ATP) released from damaged cells and from platelets undergoing exocytosis have not been clearly established. In this study we report on the effects of ATP on platelet aggregation in whole blood. Methods and Results— Aggregation, measured using a platelet-counting technique, occurred in response to ATP and was maximal at ...
Under physiological conditions, ATP is present in the extracellular space at low concentrations (1-10 nM), whereas during inflammation and tumor cell growth ATP is present in the extracellular space at high concentrations, when 5-10 mM of ATP are quickly released from cytoplasm following plasma membrane damage or membrane stretching.
Oxygen (O 2) is an essential molecule in the human body. It is the final electron acceptor in the electron transport chain, located in the mitochondria, and so has a key role in the production of aerobic energy - i.e. adenosine triphosphate (ATP). A constant supply is therefore required to tissues around the body, and this is achieved by the ...
Abstract. Metabolic homeostasis in animals depends critically on evolved mechanisms by which red blood cell (RBC) hemoglobin (Hb) senses oxygen (O 2) need and responds accordingly. The entwined regulation of ATP production and antioxidant systems within the RBC also exploits Hb-based O 2 -sensitivity to respond to various physiologic and ...
ATP is converted into cAMP, a major second messenger involved in many cellular processes, by adenylyl cyclase, a membrane-associated enzyme. In this review, we describe the role of ATP as a beneficial extracellular molecule released by healthy red blood cells (RBCs) in response to hypoxia to mediate a vasodilator signal, by oxidatively stressed ...
Yourbody has three different metabolic pathways: 1. Phosphagen system (ATP-PC system) for immediate energy. Phosphocreatine (PC) is a molecule in your muscles that can make ATP in the blink of an ...
1. Introduction. Classically, the nucleotide adenosine 5′-triphosphate (ATP) has been considered the major energy source in the cell. Through the metabolic breakdown of ingested nutrients, subsequent production of the electron carriers NADH and FADH 2 in the citric acid cycle and the transfer of electrons through the electron transport chain, the mitochondrial ATP synthase synthesize ATP in ...
ATP has a half-life of about 1 second in blood, mostly because of hydrolyzing enzymes. It is a potent signaling molecule in the vasculature and nervous system as well. You might envision an arrangement where this isn't the case, and ATP is supplied from a central organ to other parts of the body. It would likely be more inefficient, especially ...
ATP - powering the cell. ATP (adenosine triphosphate) is the energy-carrying molecule used in cells because it can release energy very quickly. Energy is released from ATP when the end phosphate ...
ATP is a major player as a signaling molecule in blood microcirculation. It is released by red blood cells (RBCs) when they are subjected to shear stresses large enough to induce a sufficient shape deformation. This prominent feature of chemical response to shear stress and RBC deformation constitutes an important link between vessel geometry, flow conditions, and the mechanical properties of ...
Abstract. Although there is an abundance of evidence which indicates that ATP is released as well as taken up by cells, the concept that ATP cannot cross the cell membrane has tended to prevail. This article reviews the evidence for the release as well as uptake of ATP by cells. The evidence presented by various investigators clearly indicates ...
The heart is an amazing organ. It starts beating about 22 days after conception and continuously pumps oxygenated red blood cells and nutrient-rich blood and other compounds like platelets throughout your body to sustain the life of your organs. Its pumping power also pushes blood through organs like the lungs to remove waste products like CO2.. This fist-sized powerhouse beats (expands and ...
Abstract. ATP is a major player as a signaling molecule in blood microcirculation. It is released by red blood cells (RBCs) when they are subjected to shear stresses large enough to induce a sufficient shape deformation. This prominent feature of chemical response to shear stress and RBC deformation constitutes an important link between vessel ...
The Donation. You arrive for your blood donation appointment. Health history and mini physical are completed. For a whole blood donation, about 1 pint of blood is collected; several small test tubes of blood are also collected for testing. Your donation, test tubes and your donor record are labeled with an identical bar code label.
Abstract. Background: Human plasma ATP concentration is reported in many studies as roughly 1000 nmol/L. The present study tested the hypothesis that the measured plasma ATP concentration is lower if ATP release from formed blood elements is inhibited during blood sample processing. A second hypothesis was that pretreatment with aspirin to ...
Red light therapy may work in skin health to: Stimulate collagen production, which gives skin its structure, strength and elasticity. Increase fibroblast production, which makes collagen. Collagen is a component of connective tissue that builds skin. Increase blood circulation to the tissue. Reduce inflammation in cells.